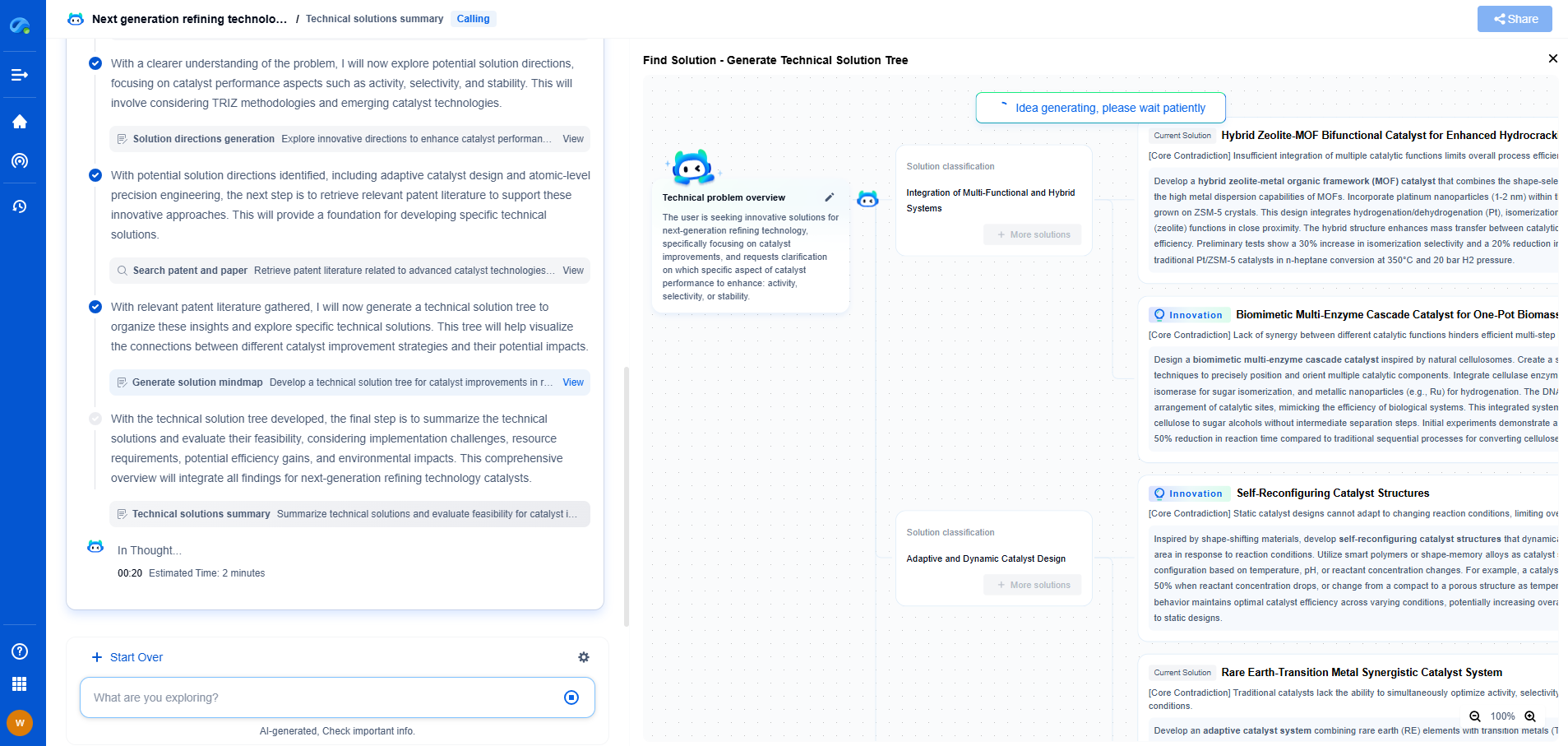
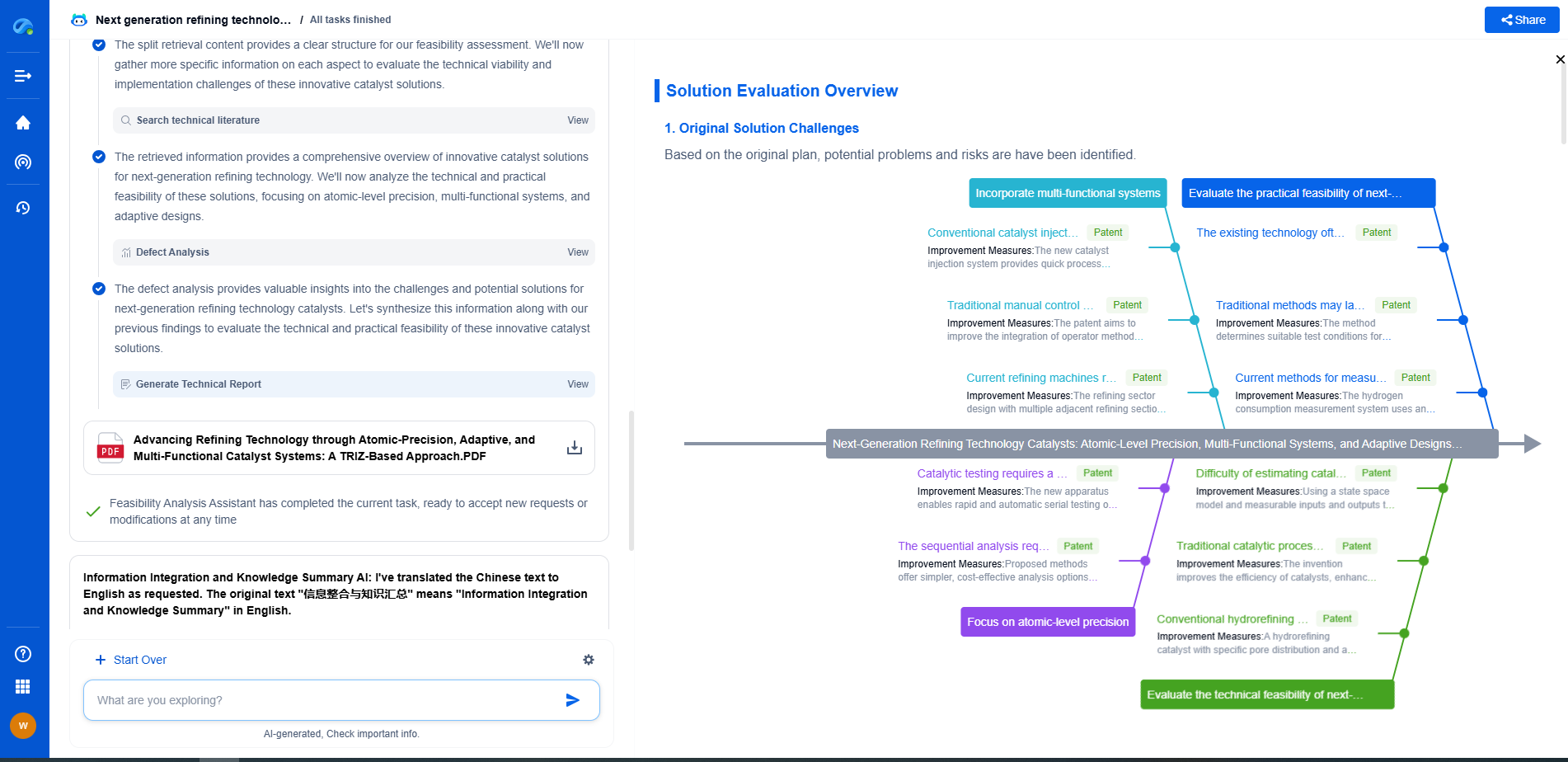
Correcting for Scattering Effects in Absorbance Measurements
JUL 15, 2025 |
In the realm of spectrophotometry, absorbance measurements are paramount for determining the concentration of analytes in various samples. However, these measurements can be significantly influenced by scattering effects, which can introduce substantial errors. Scattering occurs when particles or molecules in a sample deflect light away from its initial path, affecting the accuracy of absorbance readings. Thus, understanding and correcting these scattering effects is crucial for obtaining reliable results.
Understanding the Nature of Scattering
Light scattering can be attributed to several factors such as particle size, shape, and refractive index. Typically, scattering is more pronounced in samples containing suspended particles or those that are not completely dissolved. Rayleigh scattering occurs when particles are much smaller than the wavelength of light, while Mie scattering is observed when particles are comparable in size to the wavelength. Each type of scattering alters the path and intensity of light, thereby impacting the absorbance values.
Impact of Scattering on Absorbance Measurements
In absorbance measurements, the primary goal is to determine the amount of light absorbed by the analyte of interest. However, scattering can create an apparent increase in absorbance, as scattered light is not detected by the photodetector. This leads to an overestimation of the analyte concentration. The extent of this impact is dependent on the wavelength of light used and the physical properties of the sample, making it essential to adopt appropriate corrective measures.
Methods for Correcting Scattering Effects
1. **Baseline Correction**
One of the simplest methods to correct for scattering is baseline correction. By measuring the absorbance of a blank or reference sample that contains the same scattering particles but no analyte, one can account for the scattering contribution. This baseline is then subtracted from the sample's absorbance, yielding a corrected measurement.
2. **Dual-Wavelength Approach**
The dual-wavelength approach involves measuring absorbance at two different wavelengths. One wavelength is chosen where the analyte absorbs light, and the other is selected where it does not. The difference in absorbance between these wavelengths helps isolate the scattering effect, allowing for a more accurate determination of the analyte concentration.
3. **Mathematical Modelling**
Advanced mathematical models can be employed to deconvolute scattering effects from absorbance data. Techniques such as Kubelka-Munk theory and multivariate analysis use mathematical algorithms to separate scattering contributions from the true absorbance signal. These methods require a detailed understanding of the sample matrix and the scattering phenomenon.
4. **Use of Polarized Light**
In certain cases, using polarized light can help reduce scattering effects. Polarized light interacts differently with scattered particles compared to non-polarized light, potentially minimizing scattering contributions. This technique, however, necessitates specialized equipment and may not be suitable for all sample types.
Considerations for Minimizing Scattering
To minimize the influence of scattering in absorbance measurements, several practical considerations can be applied:
- **Sample Preparation:** Ensuring that samples are homogenous and free from large particulates can significantly reduce scattering. Filtration or centrifugation may be necessary for certain samples.
- **Choice of Wavelength:** Selecting an appropriate wavelength that minimizes scattering while maximizing analyte absorption can improve measurement accuracy.
- **Instrument Calibration:** Regular calibration of spectrophotometers with standards that mimic the sample matrix can help account for scattering effects.
Conclusion
Correcting for scattering effects in absorbance measurements is essential for ensuring the accuracy and reliability of spectrophotometric analyses. By understanding the underlying principles of light scattering and employing appropriate correction techniques, researchers and practitioners can obtain more precise and meaningful data. Ultimately, vigilance in sample preparation and method selection can greatly mitigate the impact of scattering, leading to more robust analytical outcomes.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com