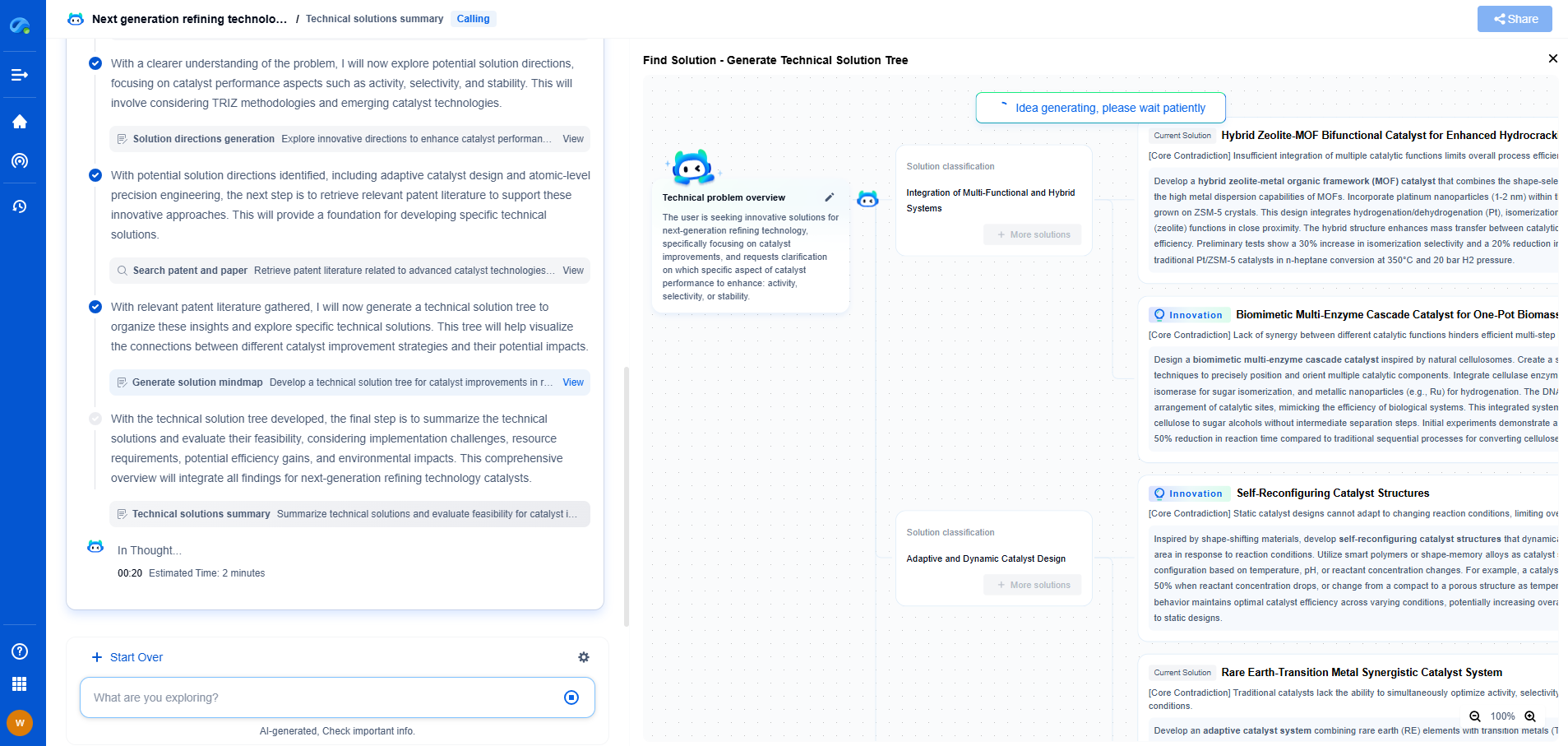
Fluorescence Spectrometers: Why Excitation and Emission Scans Differ
JUL 15, 2025 |
Fluorescence spectrometry is a powerful analytical technique used extensively in chemistry, biology, and medicine to study the properties of organic and inorganic compounds. The technique is based on the absorption and subsequent emission of light by a substance, and it allows scientists to identify and quantify various molecular species. A fluorescence spectrometer typically measures two types of spectra: excitation spectra and emission spectra. However, these scans are inherently different due to the distinct processes they represent. This blog will explore the reasons behind these differences and their implications.
The Basics of Excitation and Emission
To appreciate why excitation and emission scans differ, it's crucial to understand the fundamental processes of fluorescence. When a molecule absorbs light, it gets excited from its ground state to a higher energy state. This process is captured in the excitation spectrum, which represents the various wavelengths that can excite the molecule. Following excitation, the molecule eventually returns to its ground state, emitting light in the process. The emitted light is captured in the emission spectrum, which displays the wavelengths at which the molecule emits light.
Differences in Light Absorption and Emission
One of the primary reasons excitation and emission scans differ is due to the Stokes shift. The Stokes shift is the difference in wavelength between the peak of excitation and the peak of emission. This shift occurs because the energy absorbed by the molecule is often higher than the energy it emits. The absorbed energy puts the molecule into a higher vibrational energy level, and as it relaxes back down, some energy is lost as heat before it emits light. This energy loss results in the emission light having a longer wavelength (lower energy) compared to the absorbed light.
Spectral Bandwidth Variations
Another factor contributing to the differences between excitation and emission scans is the spectral bandwidth of the light source and detectors. In excitation spectra, the instrument typically uses a monochromator to select specific wavelengths of light that excite the sample. Meanwhile, in emission spectra, the emitted light is dispersed and detected across a range of wavelengths. These differences in detection methods can result in variations in the resolution and sensitivity of the recorded spectra, leading to discrepancies between excitation and emission scans.
Instrumental and Environmental Influences
The differences between excitation and emission spectra can also be influenced by instrumental factors and environmental conditions. For instance, the fluorescence spectrometer’s lamp intensity, detector sensitivity, and monochromator efficiency can alter the recorded spectra. Additionally, environmental factors such as temperature, solvent polarity, and pH can affect the molecular environment, impacting both the absorption and emission processes, thus leading to variations in the spectra.
Practical Applications and Considerations
Understanding the differences between excitation and emission scans is crucial for researchers who rely on fluorescence spectrometry for their studies. These differences can provide valuable information about molecular interactions, energy transfer processes, and the local environment of the fluorescent species. For instance, a significant Stokes shift is often advantageous in applications like fluorescence microscopy, as it helps to separate the excitation light from the emission, reducing background noise and improving image clarity.
Conclusion
In conclusion, while excitation and emission scans in fluorescence spectrometry arise from the same fundamental process, they differ due to the Stokes shift, spectral bandwidth variations, and instrumental and environmental influences. Recognizing and accounting for these differences is essential for accurately interpreting fluorescence spectra and making meaningful scientific conclusions. Whether exploring the dynamics of biomolecules or analyzing chemical compositions, an understanding of these spectral differences enhances the application and utility of fluorescence spectrometry in research and industry.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com