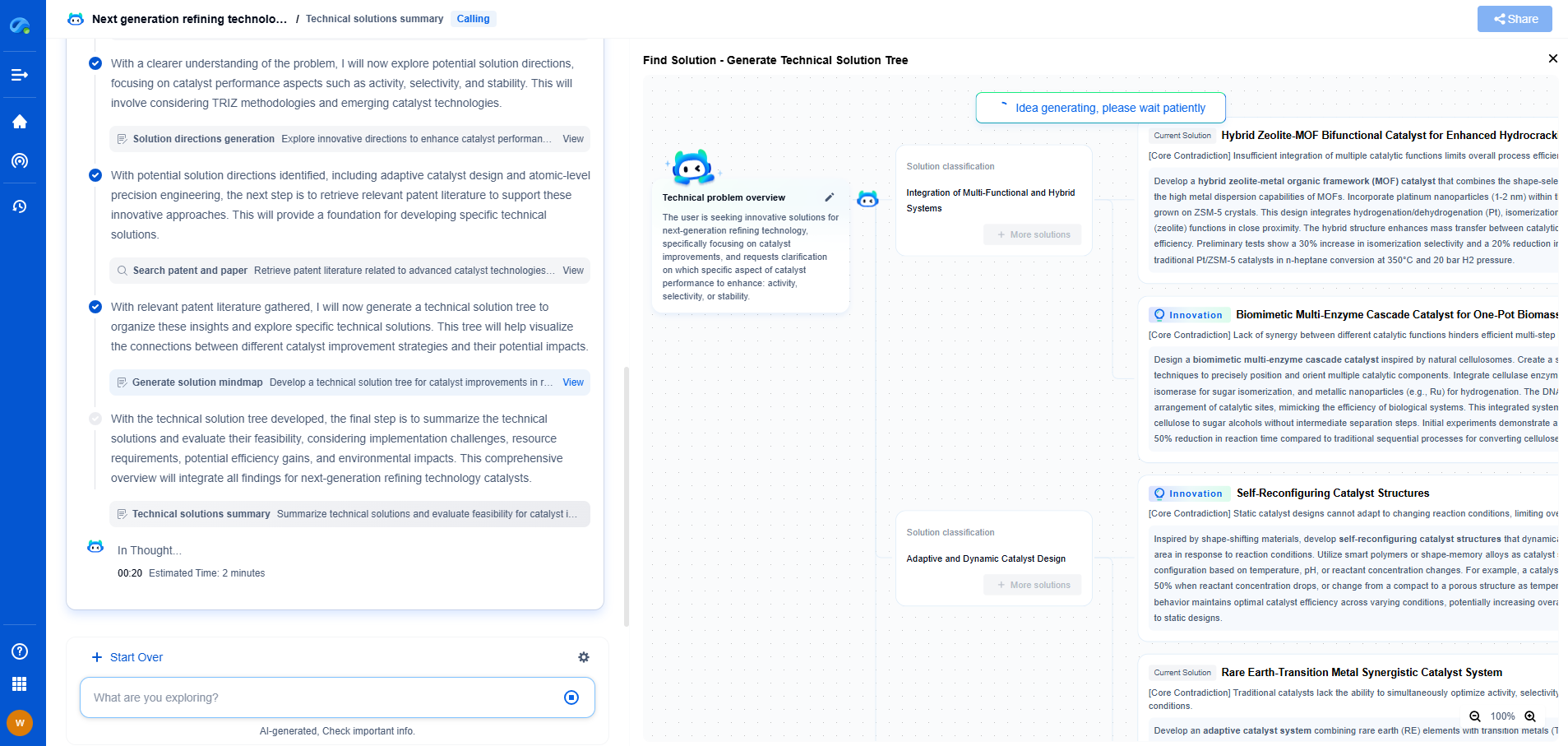
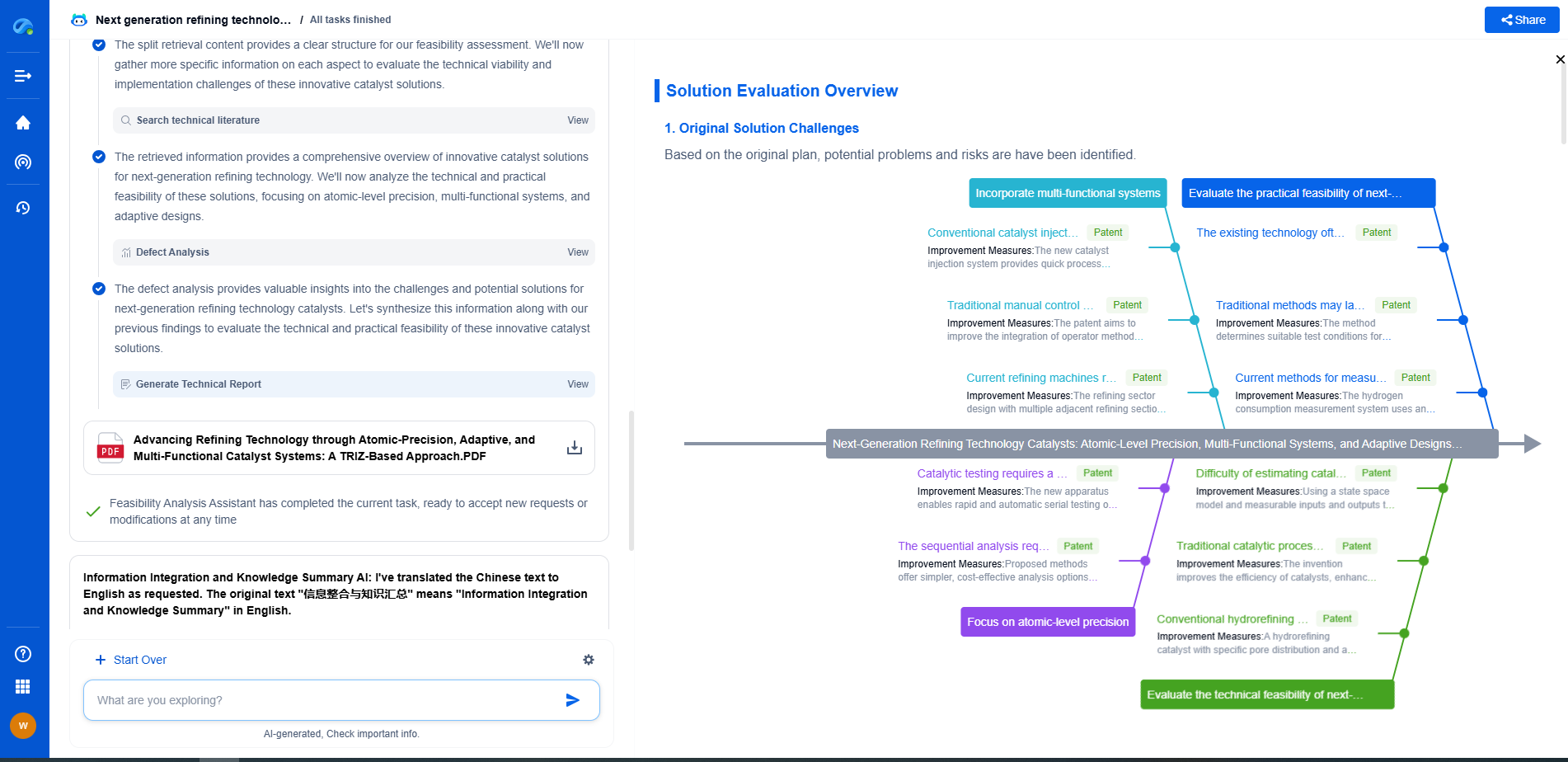
Fluorometry vs Phosphorimetry: Understanding Luminescence Techniques
JUL 15, 2025 |
When studying the properties of various chemical substances, scientists often rely on luminescence techniques to observe and measure the light emitted by a substance. Among these methods, fluorometry and phosphorimetry stand out for their specific applications and unique characteristics. Understanding these two techniques' principles, applications, and differences is essential for researchers and practitioners in fields such as chemistry, biochemistry, and environmental science.
Understanding Fluorometry
Fluorometry, also known as fluorescence spectroscopy, is a technique used to measure the intensity of fluorescent light emitted by a substance. The process begins when a molecule absorbs light at a specific wavelength, excites electrons, and subsequently emits light at a longer wavelength as it returns to its ground state. The emitted light's intensity is proportional to the concentration of the fluorescent molecules, making fluorometry an excellent tool for quantitative analysis.
One of the key advantages of fluorometry is its high sensitivity, capable of detecting low concentrations of fluorescent molecules. This sensitivity makes it particularly useful in applications such as detecting trace elements in environmental samples, analyzing biological samples for the presence of specific biomarkers, and examining the structural properties of proteins and nucleic acids. Furthermore, fluorometry offers rapid and non-destructive analysis, allowing for real-time monitoring of dynamic processes.
Exploring Phosphorimetry
Phosphorimetry, on the other hand, is related to the phenomenon of phosphorescence, where a substance emits light after absorbing it, but with a noticeable delay. This delay occurs due to the involvement of triplet state electrons, which take longer to return to their ground state compared to the singlet states involved in fluorescence. As a result, phosphorescence can last from microseconds to several hours, depending on the substance and environmental conditions.
Phosphorimetry is especially beneficial when analyzing substances that exhibit strong phosphorescent properties. Its applications extend to fields such as material science, where it is used to study the properties and lifetimes of phosphorescent materials in devices like OLEDs (organic light-emitting diodes) and glow-in-the-dark products. Additionally, phosphorimetry is useful in analyzing certain biological samples where phosphorescent markers can provide insights into cellular activities and dynamics.
Key Differences Between Fluorometry and Phosphorimetry
While both fluorometry and phosphorimetry involve the measurement of emitted light, the fundamental distinction lies in the nature of the luminescent processes they observe. Fluorometry deals with fluorescence, characterized by rapid emission following excitation. In contrast, phosphorimetry focuses on phosphorescence, where the emission process is delayed due to the involvement of triplet states.
This difference in emission timing impacts the techniques' applications and equipment requirements. Fluorometers are typically equipped with pulse or continuous wave light sources and sensitive detectors capable of capturing fast emission events. Phosphorimeters, in contrast, need to accurately measure delayed emissions and often employ different detection methodologies to handle the longer-lived phosphorescent signals.
Applications and Practical Considerations
Both techniques require careful consideration of various factors, such as the choice of excitation light source, detector sensitivity, and environmental conditions. Factors like temperature, pH, and the presence of quenching agents can significantly affect the luminescent properties of the sample. Therefore, understanding these parameters is crucial to obtaining reliable and reproducible results.
In practical terms, fluorometry is often favored for applications requiring quick analysis and high sensitivity to low concentrations. Its ability to rapidly detect changes in fluorescence intensity makes it invaluable in clinical diagnostics, where it is used to identify specific proteins or nucleic acids in patient samples. Conversely, phosphorimetry is more suited to applications where the temporal properties of emission are critical, such as in the development of materials with long-lasting luminescence.
Conclusion
Fluorometry and phosphorimetry are powerful luminescence techniques, each with its own strengths and applications. Fluorometry is known for its speed and sensitivity, making it ideal for real-time analysis of biological and environmental samples. Phosphorimetry, with its focus on delayed emissions, offers unique insights into materials science and cellular dynamics. Understanding the principles and differences between these methods is essential for selecting the right technique to meet specific research needs and achieve accurate, reliable results in the study of luminescent materials and processes.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com