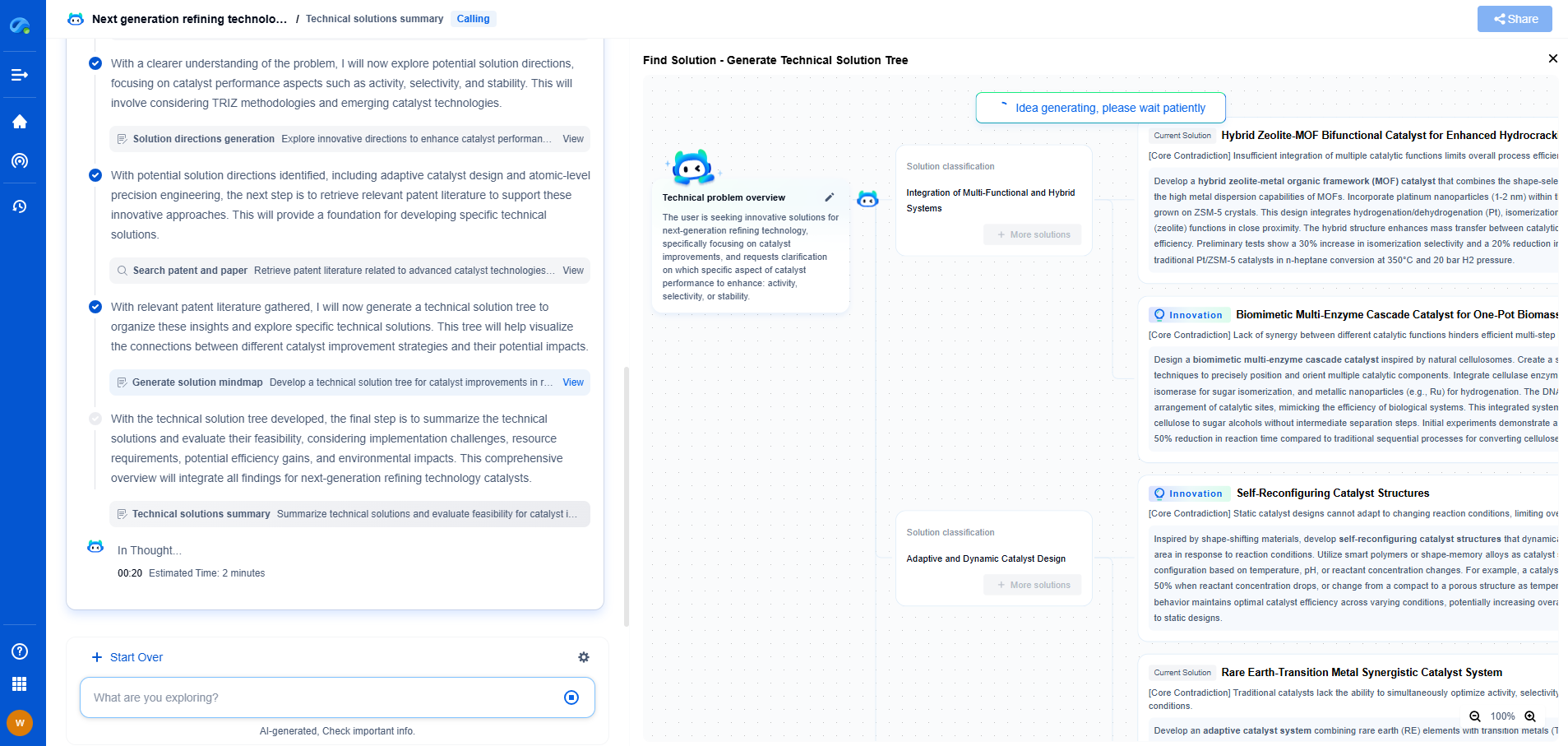
How to use traceable calibration standards in regulated environments
JUL 15, 2025 |
In regulated environments, maintaining the integrity, accuracy, and reliability of measurement instruments is crucial. One of the most effective ways to achieve this is through traceable calibration standards. These standards ensure that the measurements obtained from instruments are accurate and consistent, complying with both national and international regulations. In this blog post, we will explore the importance of traceable calibration standards and how to effectively utilize them in regulated settings.
What Are Traceable Calibration Standards?
Traceable calibration standards refer to documented calibration processes that can be linked to national or international standards through an unbroken chain of comparisons. This traceability ensures that the measurements and data produced by an instrument are accurate and consistent with global measurement benchmarks. The traceability is typically achieved through a hierarchical chain of calibrations, beginning with a primary standard maintained by a national metrology institute and extending down to the working standards used in everyday operations.
The Importance of Traceable Calibration
In regulated environments, such as pharmaceuticals, aerospace, and manufacturing, precise measurements are not just a best practice but a legal requirement. Traceable calibration standards provide confidence that the instruments are operating correctly, reducing risks associated with measurement errors. For example, in the pharmaceutical industry, incorrect measurements can lead to ineffective or even dangerous products. Therefore, traceable calibration is essential for ensuring product quality, compliance with regulations, and maintaining consumer safety.
Choosing the Right Calibration Standards
Selecting the appropriate calibration standards is a critical step in establishing a robust traceability chain. Begin by identifying the relevant regulatory requirements specific to your industry or application. Understand the level of precision necessary for your instruments and choose standards that meet or exceed these requirements. It is essential to ensure that the calibration service provider is accredited by relevant authorities, as this guarantees the traceability of their calibration practices to national or international standards.
Establishing a Calibration Schedule
Once you have selected the appropriate standards, it is important to establish a regular calibration schedule. Regular calibration ensures that your instruments continue to provide accurate and reliable measurements over time. The frequency of calibration should be determined based on factors such as the instrument's usage, the criticality of its measurements, and the manufacturer's recommendations. Maintaining a detailed record of calibration history is also necessary for traceability compliance and can assist in identifying trends or deviations in instrument performance.
Documenting Calibration Processes
In regulated environments, documentation is not just important—it is mandatory. Documenting the calibration process provides a clear audit trail and ensures compliance with regulatory standards. This includes maintaining records of calibration certificates, which should detail the procedures followed, the standards used, and the results obtained. Proper documentation serves as evidence that the instruments have been calibrated according to traceable standards and can withstand scrutiny during audits or inspections.
Training and Competency
Ensuring that staff involved in the calibration process are adequately trained is vital. Proper training enables personnel to understand the importance of traceable calibration and equips them with the skills necessary to perform calibrations accurately. Organizations should also establish competency requirements and regularly assess the performance of staff involved in calibration activities. This approach helps maintain the quality and reliability of the calibration process, further reinforcing compliance with regulatory standards.
Continuous Improvement of Calibration Practices
Finally, fostering a culture of continuous improvement within the calibration process can enhance the overall measurement reliability within an organization. Regularly reviewing and updating calibration practices to incorporate technological advancements or changes in regulatory requirements ensures that your traceability chain remains robust and effective. Engaging in benchmarking exercises and learning from industry best practices can also help identify areas for improvement.
In conclusion, in regulated environments, employing traceable calibration standards is a fundamental requirement for ensuring measurement accuracy, regulatory compliance, and product quality. By understanding and implementing these standards effectively, organizations can not only meet regulatory obligations but also enhance their reputation for reliability and excellence in their respective fields.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com