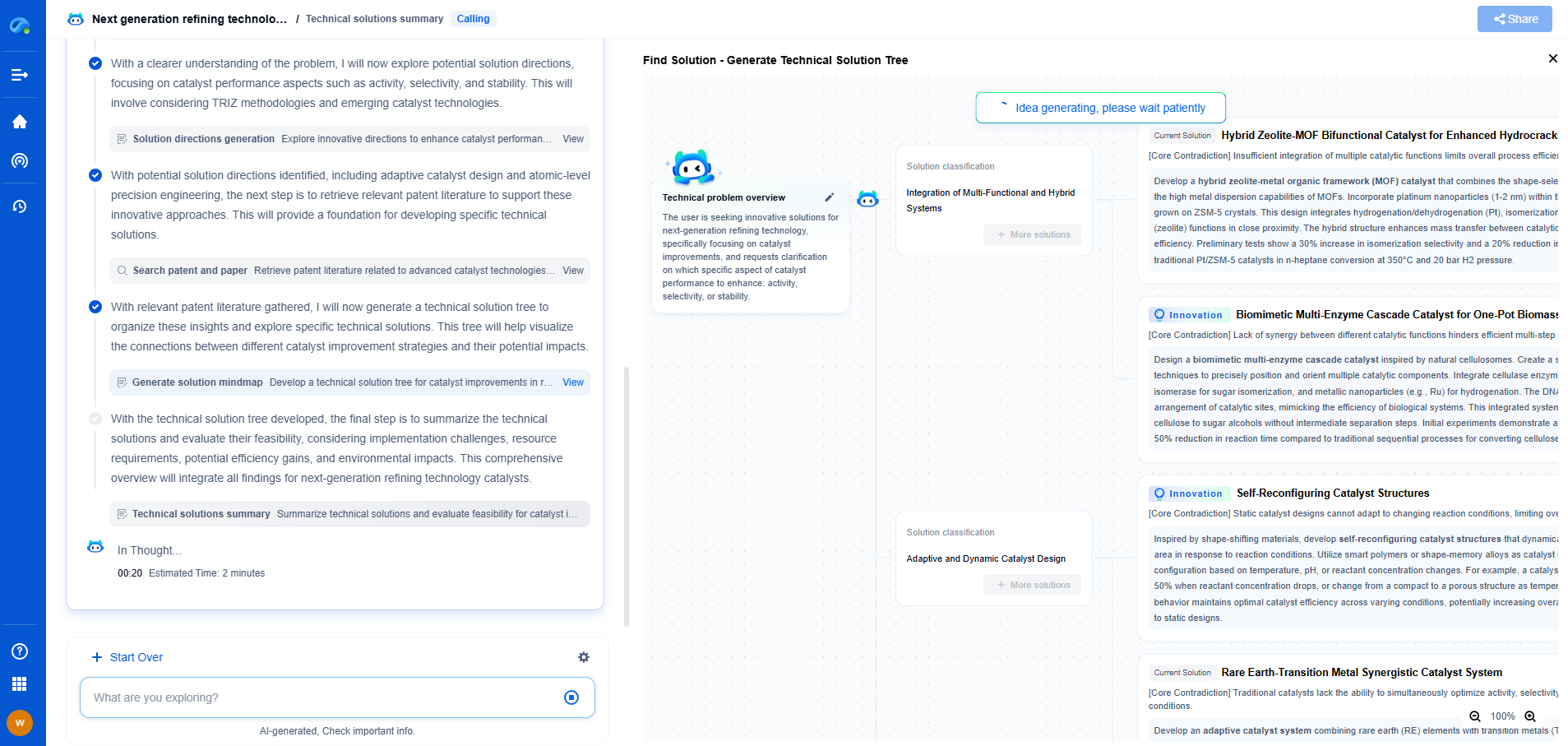
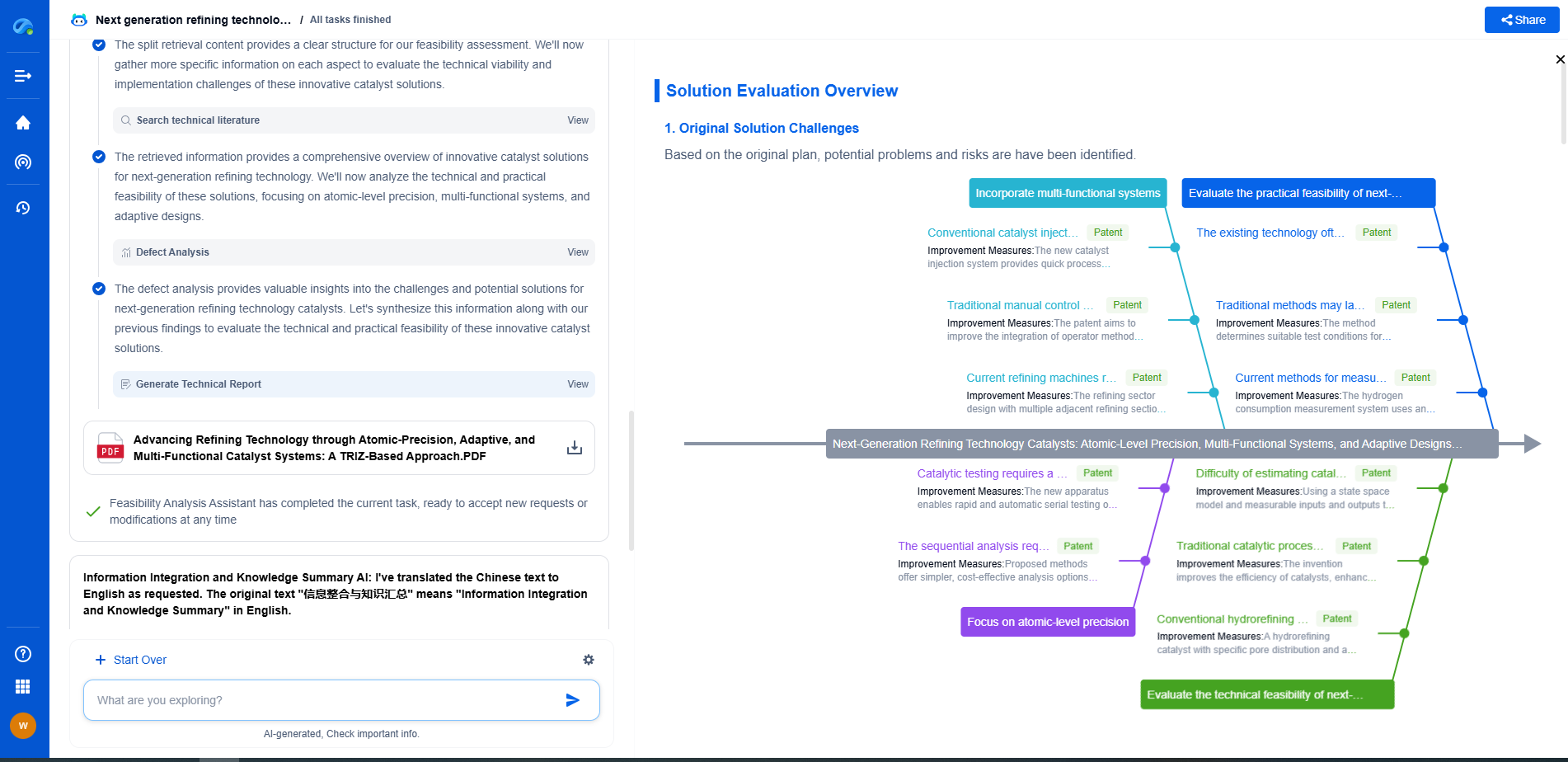
Hydrogen Diffusion Rates Through Metals: Fick's Law Applied to Storage Tanks
JUL 21, 2025 |
Hydrogen is increasingly being considered as a clean energy carrier, offering potential solutions for reducing carbon emissions. However, storing hydrogen efficiently presents numerous challenges, one of the most significant being its high diffusivity through metals. Understanding how hydrogen moves through these materials is crucial for designing effective storage systems. By applying Fick's Law, we can gain insights into the behavior of hydrogen diffusion in metal tanks used for storage.
Fick's Law: The Foundation of Diffusion Studies
Fick's Law of Diffusion is a fundamental principle that describes how particles, such as hydrogen atoms, spread over time. The law is primarily divided into two parts: Fick's First Law and Fick's Second Law.
Fick's First Law states that the diffusion flux is proportional to the concentration gradient. In simpler terms, hydrogen moves from areas of high concentration to areas of low concentration, and the rate of this movement is dependent on the difference in concentrations.
Fick's Second Law extends this idea by considering how concentration changes with time. This aspect is particularly useful in understanding transient states of diffusion, which are common in practical applications like hydrogen storage tanks.
Factors Influencing Hydrogen Diffusion in Metals
Several factors influence how hydrogen diffuses through metals, each playing a critical role in designing storage solutions.
1. **Material Properties:** The type of metal significantly affects diffusion rates. For instance, metals such as palladium and platinum are known for their high hydrogen solubility and permeability, making them less desirable for storage purposes compared to more resistant materials like aluminum or stainless steel.
2. **Temperature:** As temperature increases, so does the rate of hydrogen diffusion. This is due to increased atomic vibrations, which allow hydrogen atoms to move more freely through the metal lattice. Therefore, storage tanks must be designed to withstand operational temperatures while minimizing diffusion.
3. **Pressure:** Higher hydrogen pressures increase the concentration gradient, thus accelerating diffusion rates according to Fick's Law. Storage tanks must be engineered to handle these pressures without significant losses over time.
4. **Metal Microstructure:** The arrangement of atoms and defects within the metal can facilitate or hinder hydrogen diffusion. Grain boundaries, dislocations, and vacancies all play a role in determining diffusion pathways.
Implications for Hydrogen Storage Tanks
Given the properties of Fick's Law and the factors influencing diffusion, several implications arise for the design and operation of hydrogen storage tanks.
1. **Material Selection:** Choosing the right material is crucial. Metals with low hydrogen permeability and solubility should be prioritized to reduce diffusion rates. Alloys and composite materials can also be engineered to enhance resistance to hydrogen permeation.
2. **Tank Design:** The design must account for thermal management to control temperature-related diffusion. Insulating materials and cooling systems can help maintain operational temperatures that minimize hydrogen loss.
3. **Pressure Management:** Maintaining optimal pressure levels is essential. Tanks should be equipped with pressure regulation systems to balance internal and external pressures, reducing the concentration gradient and subsequent diffusion.
4. **Regular Maintenance and Monitoring:** Continuous monitoring for leaks and metal degradation is vital to ensure long-term storage effectiveness. Advanced sensors and inspection technologies can help detect early signs of increased diffusion rates.
Concluding Thoughts
Hydrogen diffusion through metals poses a significant challenge in the quest for efficient hydrogen storage solutions. By applying Fick's Law, engineers and scientists can better understand and mitigate the factors that contribute to unwanted hydrogen loss. Through careful material selection, innovative tank design, and diligent maintenance, the promise of hydrogen as a clean energy carrier can be more fully realized. As the energy landscape continues to evolve, addressing these fundamental challenges will be crucial for the sustainable adoption of hydrogen technologies.
As clean energy and decarbonization drive new breakthroughs in hydrogen storage, CO₂ transport, and alternative gas carriers, keeping pace with technical trends and patent activity is critical to staying competitive.
Patsnap Eureka helps innovators in compressed gas storage, high-pressure tank design, gas sensor systems, and pipeline materials accelerate research by offering instant, AI-powered insights into global patents, related technologies, and emerging white spaces.
🚀 Bring speed, precision, and strategic foresight to your innovation and IP decision-making in the gas transport sector—try Eureka today and unlock a smarter path forward.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com