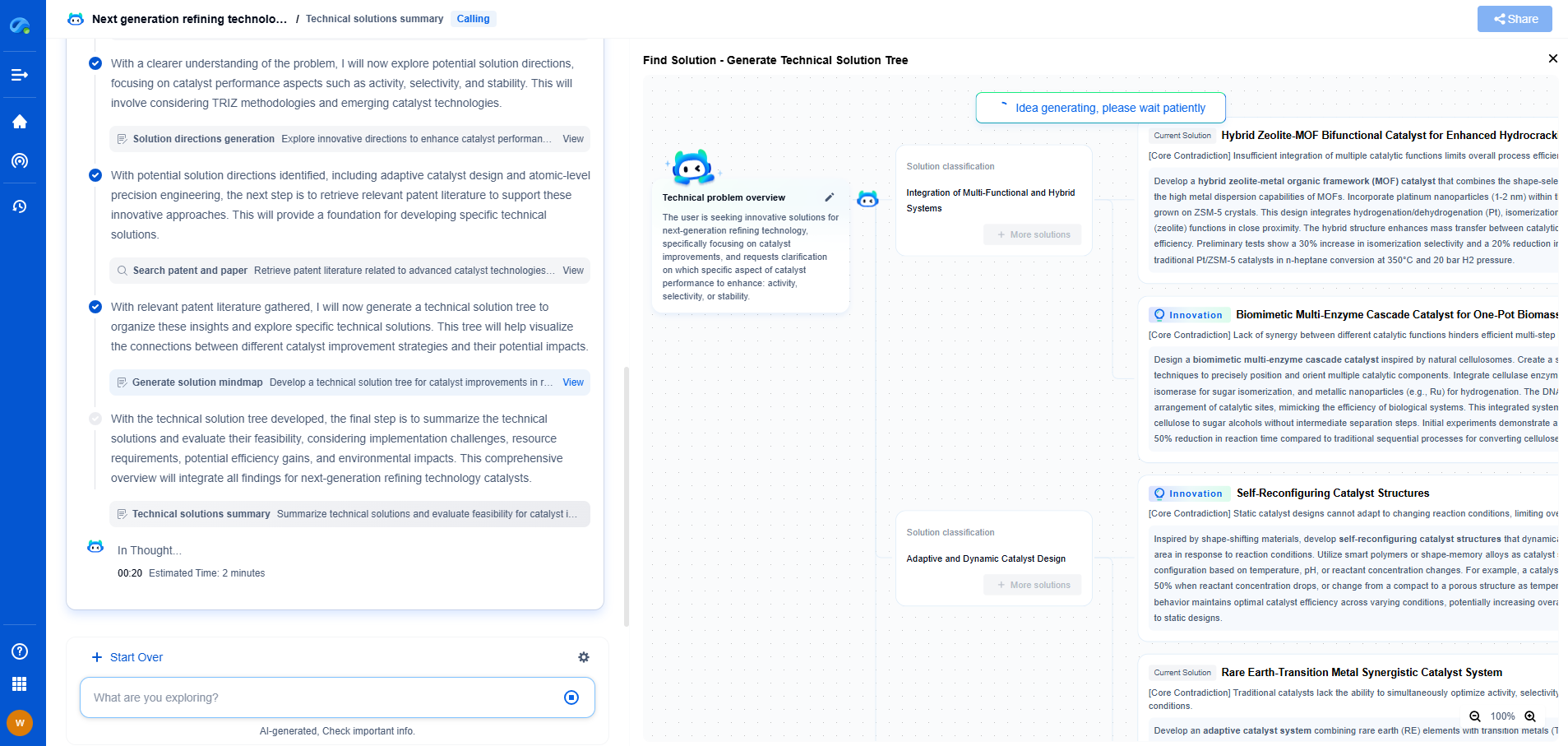
Infrared Absorption Spectroscopy: How IR Light Measures Molecular Vibrations
JUL 15, 2025 |
Infrared absorption spectroscopy is a powerful analytical technique used to identify and study chemical compounds. It is an essential tool in chemistry and biochemistry for determining molecular structure, composition, and dynamics. Unlike other spectroscopic methods, infrared spectroscopy focuses on the interaction between infrared light and matter, specifically measuring the vibrations of molecules.
The Basics of Molecular Vibrations
Molecular vibrations are the periodic movements of atoms within molecules. They occur because the atoms are constantly in motion, and these movements can be influenced by various factors, including temperature and molecular environment. In infrared spectroscopy, we are particularly interested in vibrational modes, which are specific patterns of movement that occur at characteristic frequencies.
Molecules can vibrate in several ways: stretching, bending, twisting, and wagging. Each type of vibration occurs at a different frequency, and these frequencies can be detected through infrared spectroscopy. The energy associated with these vibrations is typically within the range of infrared light.
How Infrared Spectroscopy Works
Infrared spectroscopy works by passing infrared light through a sample and measuring the absorbed wavelengths. When infrared light interacts with a molecule, certain frequencies of light are absorbed and converted into molecular vibrations. The absorbed frequencies correspond to the vibrational energies of the bonds within the molecule.
The resulting spectrum is a graph of infrared light intensity versus frequency. Peaks in the spectrum indicate specific frequencies at which the molecule absorbs infrared light, corresponding to different vibrational modes. By analyzing these peaks, scientists can determine the types of chemical bonds present and gain insights into the molecular structure.
The Role of Functional Groups
Functional groups are specific groups of atoms within molecules that have distinct chemical properties and reactivity. In infrared spectroscopy, functional groups play a crucial role because they have characteristic absorption frequencies. For instance, hydroxyl groups (-OH) typically absorb infrared light at a specific frequency, providing a "fingerprint" that helps identify the presence of alcohols or phenols in a sample.
By comparing the observed spectrum with known reference spectra, scientists can identify functional groups within a compound and infer its structure. This makes infrared spectroscopy invaluable for characterizing organic compounds and determining their functional groups.
Applications of Infrared Spectroscopy
Infrared spectroscopy has a wide range of applications across various scientific fields. In chemistry, it is used to identify compounds, analyze mixtures, and study reaction kinetics. In pharmaceuticals, it helps in the quality control of drug formulations by ensuring the correct composition and detecting impurities.
In environmental science, infrared spectroscopy monitors atmospheric gases and pollutants. It plays a significant role in studying climate change by measuring greenhouse gases like carbon dioxide and methane. Additionally, this technique is used in forensic science for analyzing substances found at crime scenes.
Advancements and Future Directions
Technological advancements continue to enhance the capabilities of infrared spectroscopy. The development of Fourier-transform infrared (FTIR) spectroscopy has improved the speed and accuracy of measurements. FTIR allows for the simultaneous collection of data over a wide range of frequencies, making it more efficient than traditional methods.
Looking ahead, the integration of infrared spectroscopy with other analytical techniques, such as mass spectrometry and nuclear magnetic resonance, will provide even deeper insights into molecular structures and dynamics. These advancements will expand its applications in fields such as materials science and biomedical research.
Conclusion
Infrared absorption spectroscopy is a fundamental tool for understanding molecular vibrations and characterizing chemical compounds. By measuring the interaction between infrared light and molecules, scientists can determine molecular structure and composition with high precision. Its wide range of applications and ongoing advancements ensure that infrared spectroscopy will remain a cornerstone of scientific research and analysis for years to come.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com