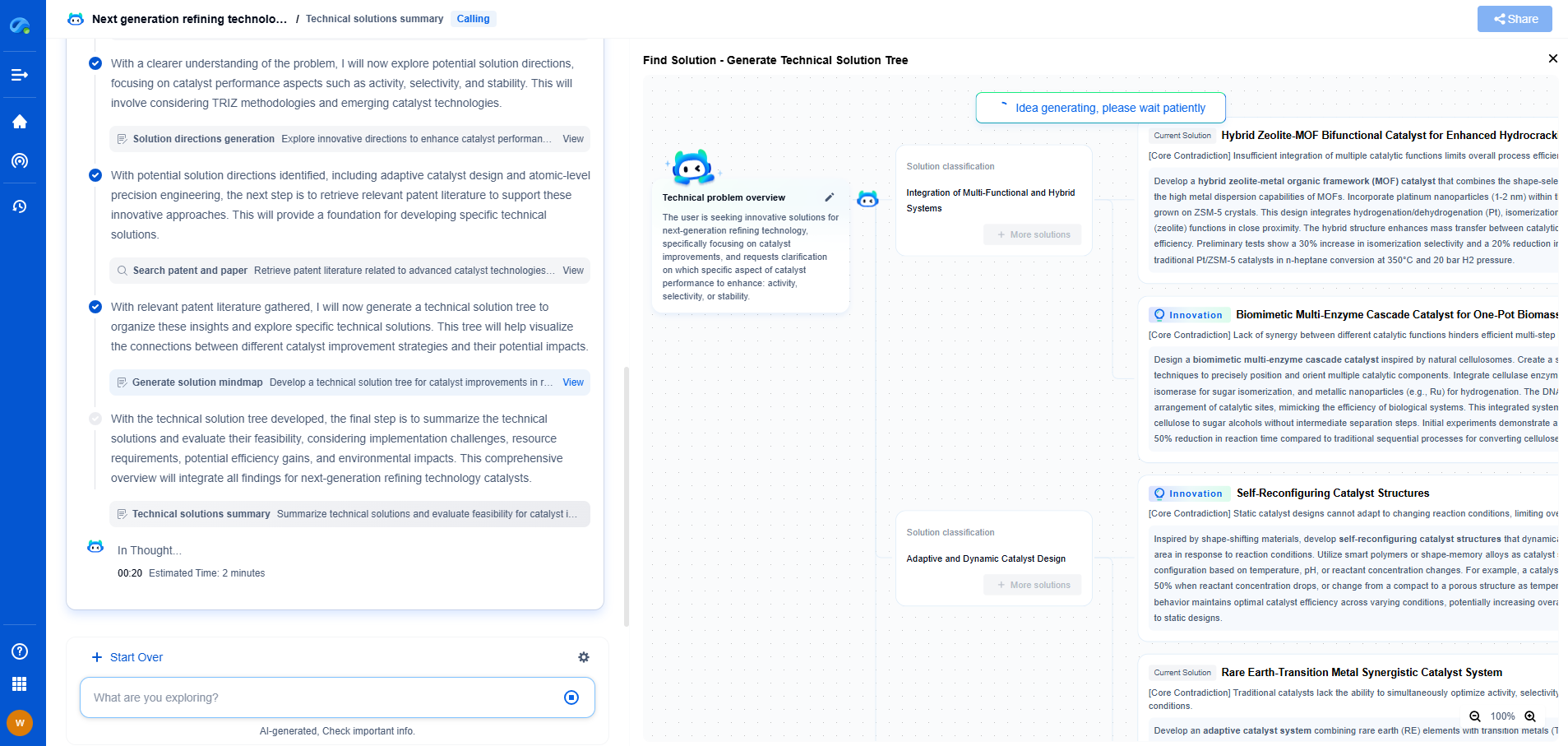
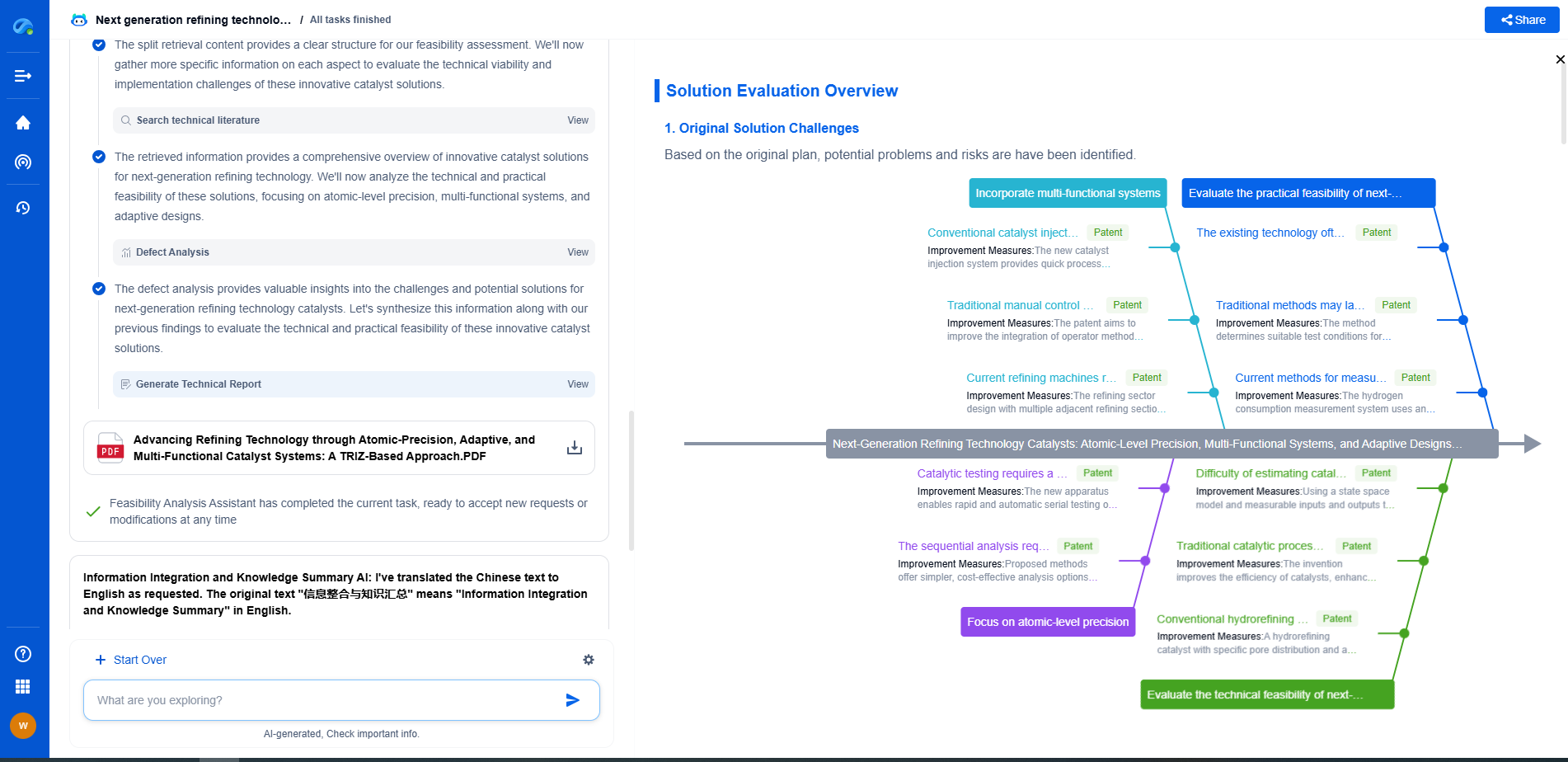
Inline NIR vs UV-Vis for Pharmaceutical Blend Uniformity Monitoring
JUL 15, 2025 |
In the pharmaceutical industry, ensuring the uniform distribution of active ingredients within a blend is critical for product efficacy and safety. Blend uniformity monitoring is an essential process in the manufacture of solid dosage forms, such as tablets and capsules, guaranteeing that each dosage form contains the intended therapeutic effect. Traditionally, this process has been carried out using various techniques, but the advent of advanced spectroscopic methods has revolutionized monitoring practices. Among these, Inline Near-Infrared (NIR) spectroscopy and Ultraviolet-Visible (UV-Vis) spectroscopy have emerged as prominent contenders. This blog explores their applications, advantages, and limitations in pharmaceutical blend uniformity monitoring.
Understanding Inline NIR Spectroscopy
Inline NIR spectroscopy is a nondestructive analytical technique that utilizes the near-infrared region of the electromagnetic spectrum to analyze pharmaceutical blends. It provides real-time data, allowing for immediate adjustments in the manufacturing process, which is crucial for maintaining blend uniformity. NIR spectroscopy excels in measuring the chemical and physical properties of materials, making it an excellent tool for monitoring blend uniformity.
Advantages of Inline NIR
One of the most significant advantages of inline NIR is its ability to provide continuous, real-time monitoring. This capability allows for immediate detection of any deviations in blend uniformity, thereby minimizing the risk of producing out-of-specification products. Moreover, NIR spectroscopy is non-invasive and does not require sample preparation, reducing time and resource consumption. Its ability to penetrate materials also enables the analysis of samples with varying particle sizes and densities, offering a comprehensive understanding of the blend's composition.
Challenges with NIR
Despite its advantages, inline NIR spectroscopy has limitations. It can be sensitive to changes in moisture content and temperature, which might affect the accuracy of the results. Additionally, the technique requires extensive calibration processes to ensure accurate predictions, which can be time-consuming and complex. Nonetheless, with proper calibration and control strategies, these challenges can be mitigated.
Exploring UV-Vis Spectroscopy
UV-Vis spectroscopy, on the other hand, involves measuring the absorption of ultraviolet or visible light by a sample. This technique is widely used in the pharmaceutical industry for quantifying active pharmaceutical ingredients (APIs) and ensuring blend uniformity. Unlike NIR, UV-Vis spectroscopy typically requires sample extraction and preparation, which can be a limiting factor for inline applications.
Advantages of UV-Vis
The primary advantage of UV-Vis spectroscopy is its high sensitivity to specific components, particularly APIs. This sensitivity allows for precise quantification of these components, ensuring accurate monitoring of blend uniformity. Additionally, UV-Vis spectroscopy is relatively simple to operate and requires less extensive calibration compared to NIR.
Limitations of UV-Vis
However, UV-Vis spectroscopy is not without its limitations. The requirement for sample preparation can be a significant drawback, as it introduces the potential for human error and delays in obtaining results. Furthermore, UV-Vis is less effective in analyzing complex mixtures with multiple components, limiting its applicability in certain pharmaceutical formulations.
Comparative Analysis: NIR vs UV-Vis
When comparing inline NIR and UV-Vis spectroscopy for pharmaceutical blend uniformity monitoring, several factors must be considered. NIR spectroscopy offers the advantage of real-time, non-invasive analysis, making it suitable for continuous monitoring during the manufacturing process. It is particularly effective for blends with multiple components, providing a comprehensive overview of the blend's composition.
In contrast, UV-Vis spectroscopy, while highly sensitive and precise for specific APIs, may be less practical for inline applications due to its requirement for sample extraction and preparation. However, its simplicity and accuracy in quantifying specific components make it an invaluable tool for confirming blend uniformity in laboratory settings.
Conclusion
In conclusion, both inline NIR and UV-Vis spectroscopy have distinct roles in pharmaceutical blend uniformity monitoring. The choice between these techniques depends on the specific requirements of the manufacturing process, including the complexity of the blend, the need for real-time monitoring, and practical considerations such as calibration and sample preparation. By understanding the strengths and limitations of each method, pharmaceutical manufacturers can optimize their blend uniformity monitoring processes, ensuring the production of high-quality, effective pharmaceutical products.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com