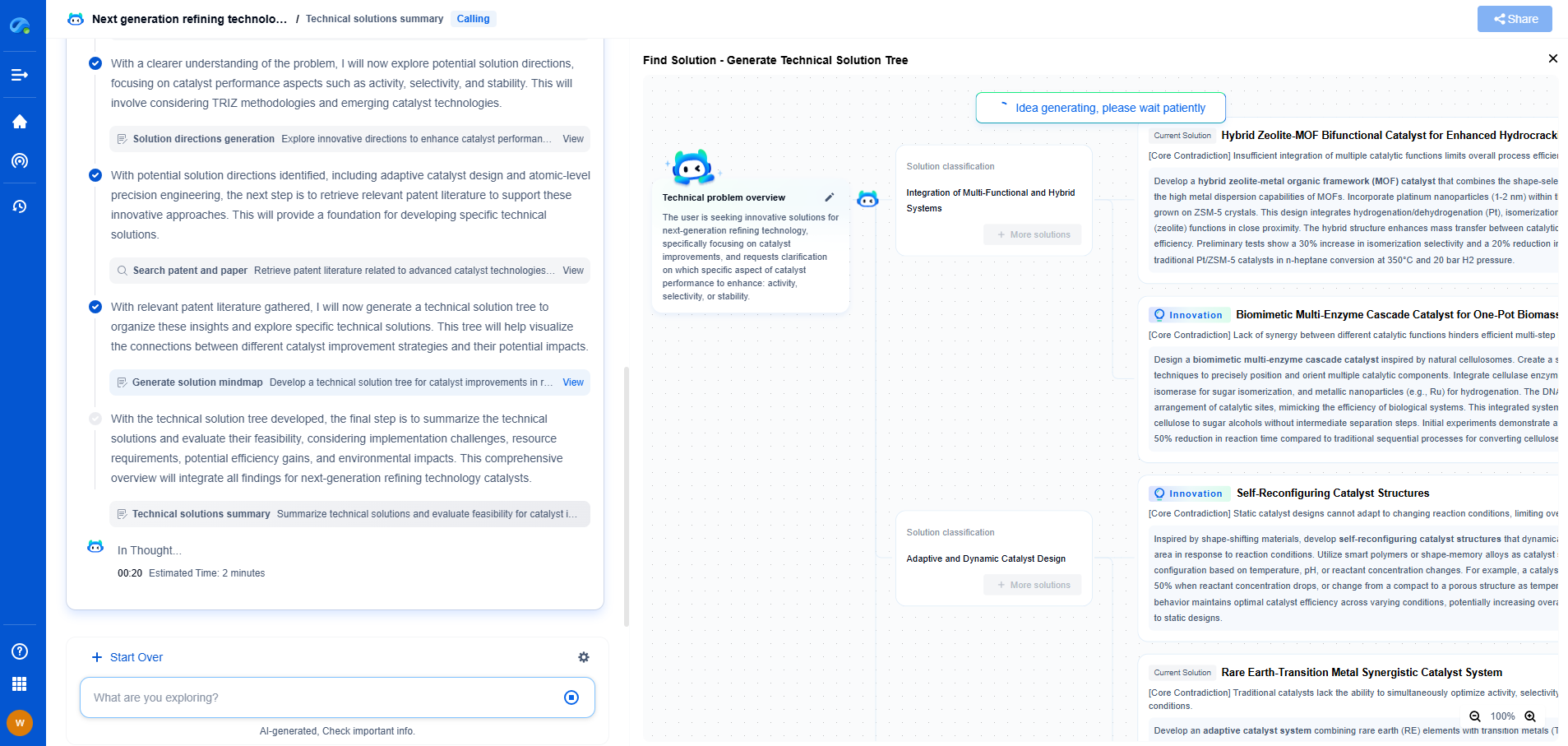
ISO standards for medical robots: What you need to know
JUN 26, 2025 |
The rapid advancement of technology in the healthcare sector has paved the way for medical robots, transforming the landscape of patient care, surgical procedures, and rehabilitation. As these robots become increasingly integral to healthcare systems, ensuring their safety, efficacy, and quality through standardized processes is crucial. This is where ISO standards come into play, providing a framework to guide the development and deployment of medical robots.
Understanding ISO Standards
The International Organization for Standardization (ISO) is an independent, non-governmental international body that develops and publishes standards to ensure the quality, safety, and efficiency of products and services. ISO standards are essential for medical robots as they help manufacturers and healthcare providers deliver reliable and safe robotic solutions. These standards cover various aspects, from design and manufacturing to risk management and quality assurance.
Key ISO Standards Relevant to Medical Robots
1. ISO 13485 - Quality Management Systems
ISO 13485 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and regulatory requirements. This standard is crucial for manufacturers of medical robots, as it ensures that the devices are produced under stringent quality controls and meet all necessary safety requirements.
2. ISO 14971 - Risk Management
ISO 14971 provides a framework for the application of risk management to medical devices. It outlines processes for identifying hazards, estimating and evaluating risks, controlling these risks, and monitoring the effectiveness of control measures. For medical robots, this standard is vital to minimize the potential risks associated with their use, ensuring patient and user safety.
3. ISO/TS 15066 - Collaborative Robots
ISO/TS 15066 is a technical specification that focuses on collaborative robots, which are designed to work alongside humans. This standard provides guidelines for the safe interaction between humans and robots, detailing the limits for robot speed and force to prevent injuries. In the medical field, where collaborative robots are often used in surgeries and rehabilitation, adhering to these guidelines is essential for maintaining a safe environment.
4. ISO 60601 - Medical Electrical Equipment
This standard deals with the safety and essential performance of medical electrical equipment. It is particularly relevant for medical robots that involve electrical components, ensuring that they function safely and effectively in medical environments. Compliance with ISO 60601 helps prevent electrical hazards and ensures the reliable operation of robotic systems in healthcare settings.
The Importance of Compliance
Compliance with ISO standards is not just about meeting international requirements; it's about ensuring the highest level of safety and quality for medical devices. Manufacturers who adhere to these standards can enhance their reputation and competitiveness in the market. For healthcare providers, using ISO-compliant robots means reduced risk of malfunction or patient harm, leading to improved patient outcomes and trust in robotic technology.
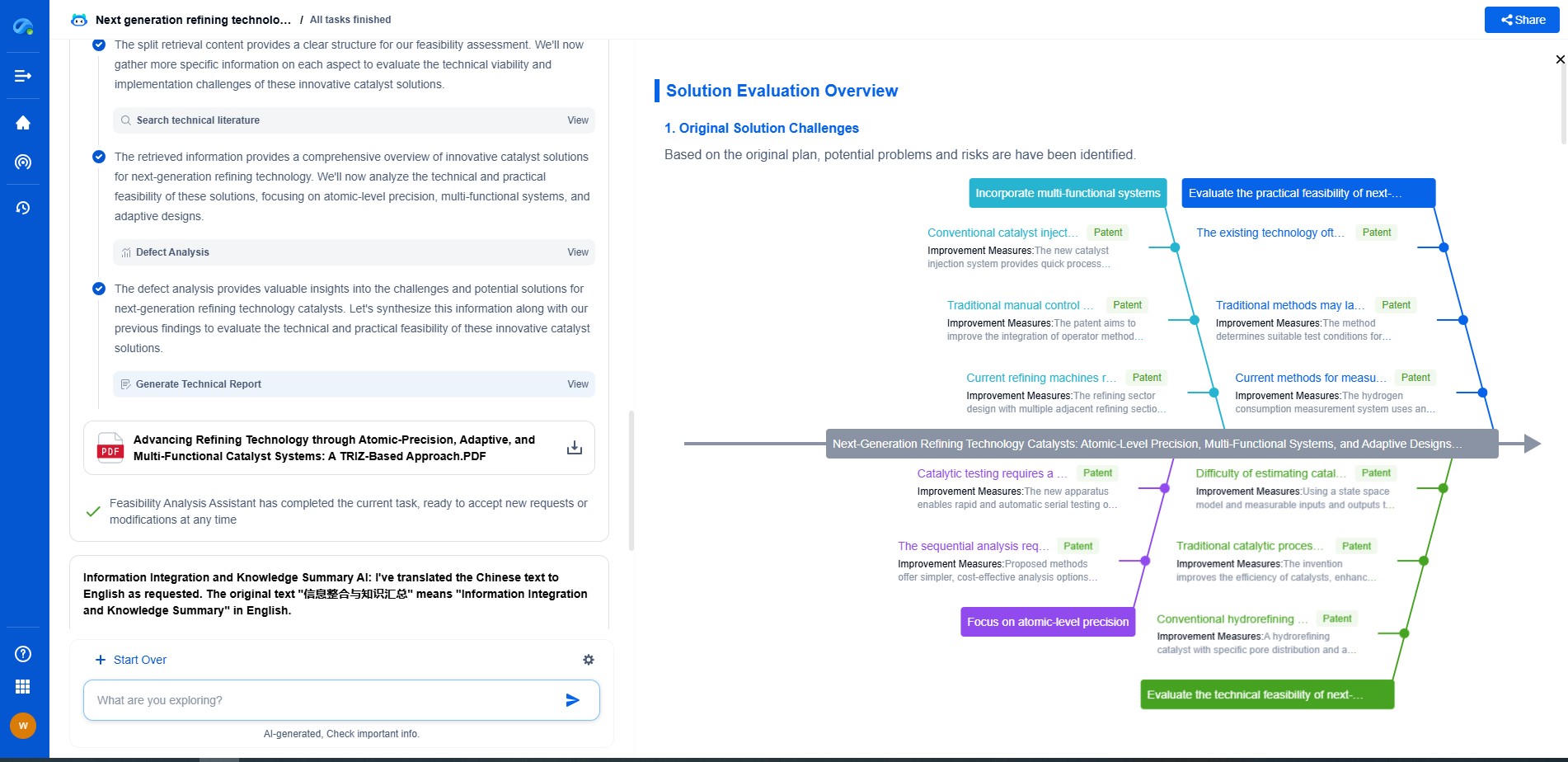
Challenges in Implementing ISO Standards
While the benefits of ISO standards are evident, implementing them can pose challenges. The development and certification process can be time-consuming and costly, especially for smaller companies. Additionally, keeping up with changes and updates to these standards requires ongoing commitment and resources. However, the long-term benefits of compliance, in terms of safety, quality, and marketability, often outweigh these challenges.
Conclusion
As medical robots continue to revolutionize healthcare, adherence to ISO standards will remain critical in ensuring that these innovations are safe, effective, and of the highest quality. By understanding and implementing these standards, manufacturers and healthcare providers can contribute to a safer healthcare environment, ultimately benefiting both patients and practitioners. As the field of medical robotics evolves, staying informed about ISO standards and their implications will be essential for all stakeholders involved.
Ready to Redefine Your Robotics R&D Workflow?
Whether you're designing next-generation robotic arms, optimizing manipulator kinematics, or mining patent data for innovation insights, Patsnap Eureka, our cutting-edge AI assistant, is built for R&D and IP professionals in high-tech industries, is built to accelerate every step of your journey.
No more getting buried in thousands of documents or wasting time on repetitive technical analysis. Our AI Agent helps R&D and IP teams in high-tech enterprises save hundreds of hours, reduce risk of oversight, and move from concept to prototype faster than ever before.
👉 Experience how AI can revolutionize your robotics innovation cycle. Explore Patsnap Eureka today and see the difference.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com