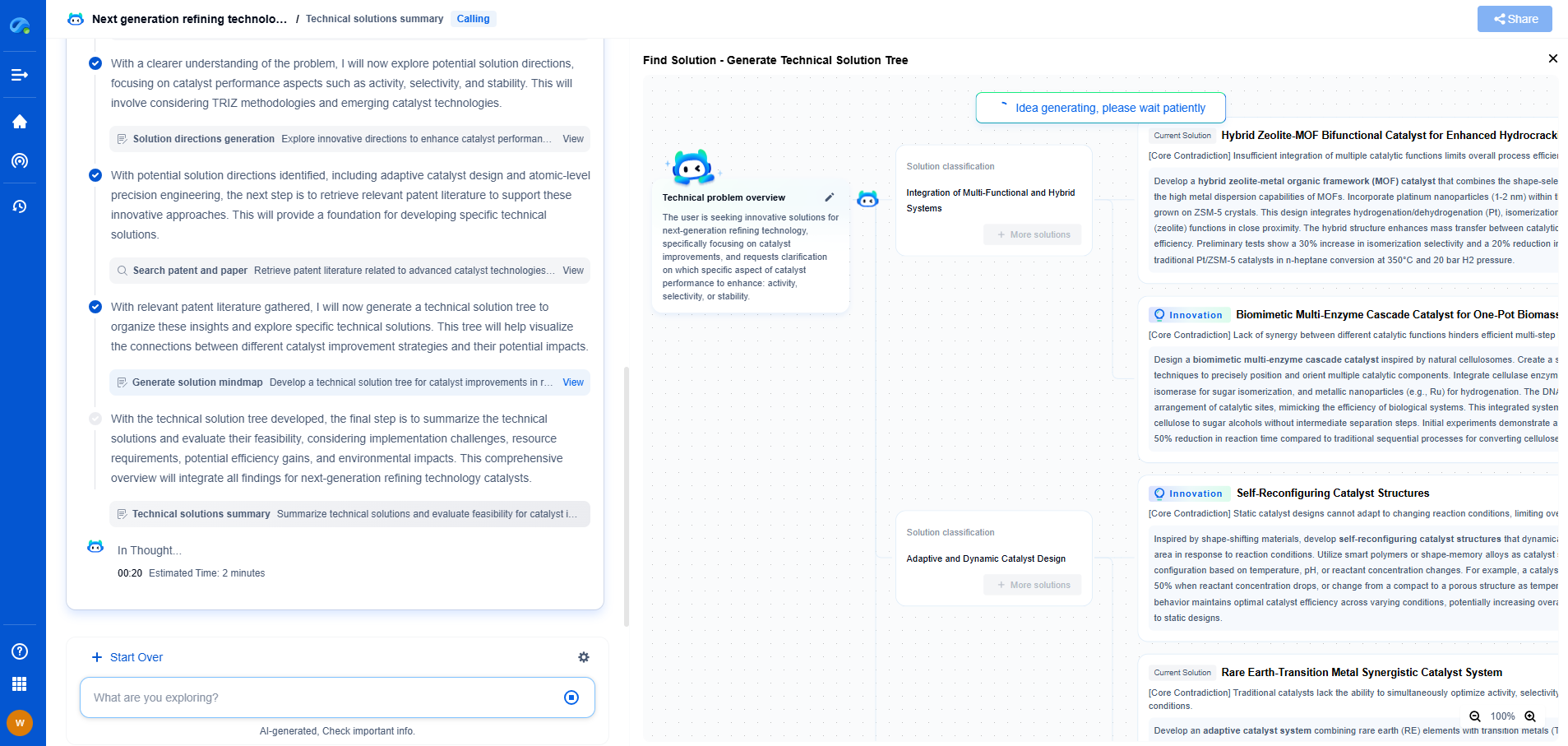
Medical Device Measurement Displays: FDA Human Factors Requirements
JUL 17, 2025 |
The design and development of medical devices are subject to stringent regulations to ensure safety and effectiveness. Among these regulations, human factors engineering (HFE) plays a crucial role, especially in the context of measurement displays. The U.S. Food and Drug Administration (FDA) emphasizes the application of human factors to minimize user error and enhance device usability. This article delves into the FDA's human factors requirements for medical device measurement displays, providing insights into the implications for device manufacturers.
The Importance of Human Factors Engineering
Human factors engineering, also known as ergonomics, is the discipline focused on understanding the interactions between humans and other system elements. In the realm of medical devices, HFE aims to optimize device design to accommodate the users' capabilities and limitations, ultimately reducing risks of errors that could compromise patient safety.
FDA's Perspective on Human Factors
The FDA recognizes the significance of human factors in medical device design and has established guidelines to ensure that devices are user-friendly and less prone to operational errors. The agency's guidance documents highlight the necessity of integrating human factors into the design process from the early stages of product development.
Key Human Factors Considerations for Measurement Displays
1. Understandability and Clarity: Measurement displays on medical devices must present information clearly and understandably. Users should be able to comprehend the data without confusion, ensuring accurate interpretation and effective decision-making.
2. Readability and Visibility: The text, numbers, and graphics on displays should be easily readable under various lighting conditions. Factors such as font size, color contrast, and display brightness must be carefully considered to enhance visibility and prevent misinterpretation.
3. Consistency and Standardization: Consistent use of symbols, units, and color codes across different devices minimizes user confusion. Standardizing these elements in line with industry norms facilitates quicker user familiarization and reduces training time.
4. Feedback and Interaction: Devices should provide immediate and intuitive feedback to users upon their interactions with the display. This feedback helps confirm correct actions and can alert users to potential errors, enhancing operational confidence and accuracy.
5. Error Prevention and Recovery: The design should incorporate features that prevent common user errors and offer pathways for error recovery. This might include warnings for out-of-range values or prompts to verify critical actions, thereby reducing the likelihood of incorrect operation.
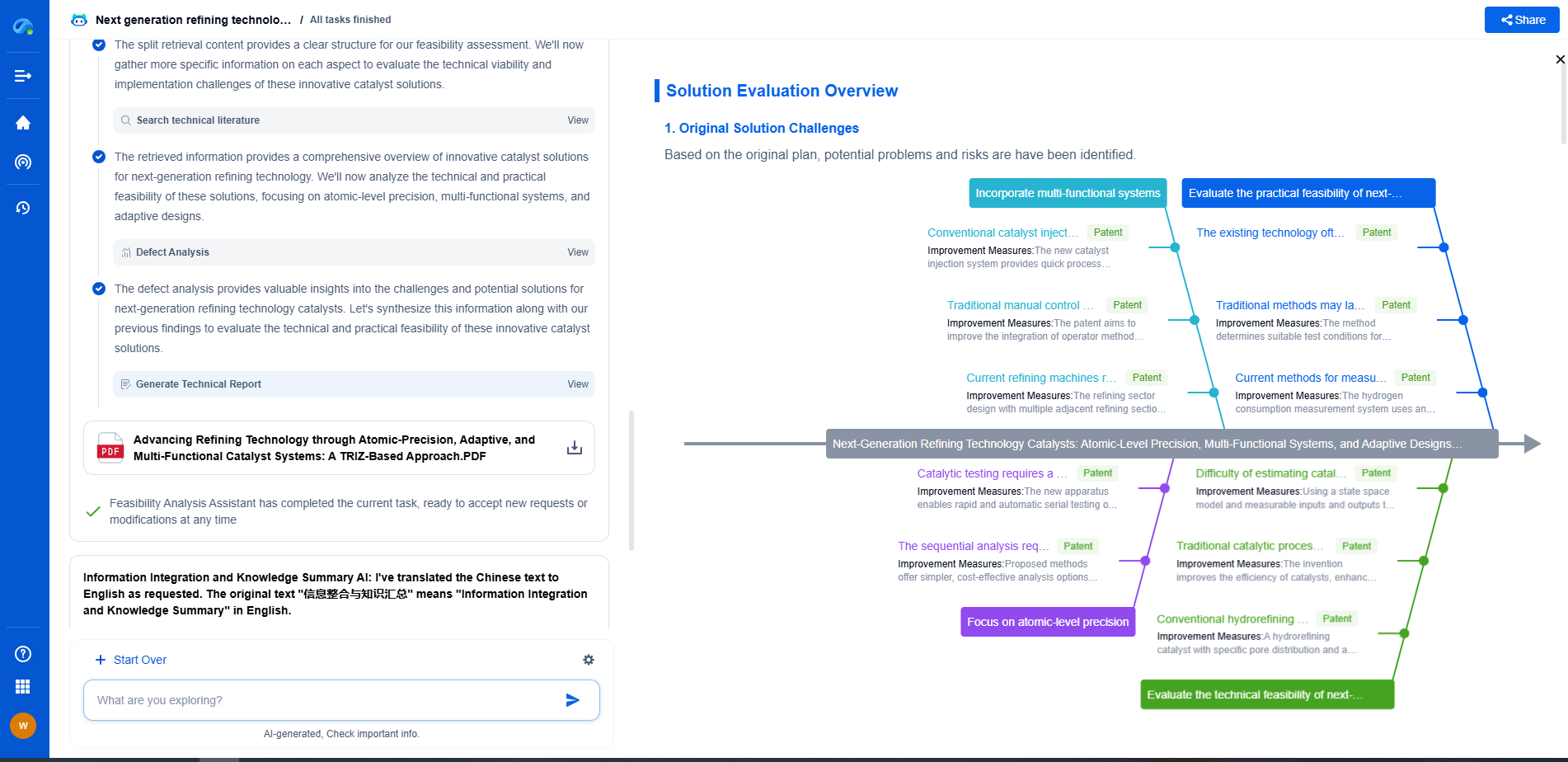
Testing and Validation of Human Factors
To ensure compliance with FDA guidelines, manufacturers must conduct thorough human factors testing throughout the device development process. This involves usability testing with representative users in realistic scenarios to identify potential issues and validate that the display design meets user needs.
Documentation and Reporting
The FDA requires comprehensive documentation and reporting of human factors activities. This documentation should outline the design rationale, testing methods, results, and any modifications made in response to usability findings. Adequate documentation not only facilitates regulatory approval but also serves as a valuable resource for post-market surveillance.
Challenges and Opportunities for Manufacturers
While adhering to FDA human factors requirements can be challenging, it also presents opportunities for manufacturers to enhance their competitive edge. Devices with superior usability are likely to gain higher user acceptance, leading to better market performance and increased trust among healthcare professionals.
Conclusion
The integration of human factors engineering into the design of medical device measurement displays is crucial for ensuring both regulatory compliance and user satisfaction. By prioritizing understandability, readability, consistency, feedback, and error prevention, manufacturers can create devices that not only meet FDA requirements but also enhance the overall quality of patient care. Through diligent testing, validation, and documentation, the goal of safer and more effective medical devices becomes increasingly attainable.
Whether you’re developing multifunctional DAQ platforms, programmable calibration benches, or integrated sensor measurement suites, the ability to track emerging patents, understand competitor strategies, and uncover untapped technology spaces is critical.
Patsnap Eureka, our intelligent AI assistant built for R&D professionals in high-tech sectors, empowers you with real-time expert-level analysis, technology roadmap exploration, and strategic mapping of core patents—all within a seamless, user-friendly interface.
🧪 Let Eureka be your digital research assistant—streamlining your technical search across disciplines and giving you the clarity to lead confidently. Experience it today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com