Medical Equipment Leakage Testing: IEC 60601 Safety Requirements Explained
JUL 9, 2025 |
Medical equipment is crucial in diagnosing, monitoring, and treating patients, making safety paramount. Among the various safety standards that govern medical devices, IEC 60601 stands out as one of the most comprehensive. A critical aspect of these standards is leakage testing, which ensures that the electrical components of medical devices do not pose risks to patients or healthcare providers. This article delves into the essentials of medical equipment leakage testing and how it aligns with IEC 60601 safety requirements.
What is Leakage Current?
Leakage current refers to the small amounts of electrical current that may pass through or leak from a medical device under normal or fault conditions. While often minimal, this current can be hazardous if it flows through the patient's body, potentially leading to shocks, burns, or other injuries. Therefore, understanding and controlling leakage current is vital in the design and testing of medical devices.
IEC 60601: A Benchmark for Safety
The IEC 60601 standard is an internationally recognized benchmark that sets stringent regulations for the safety and performance of medical electrical equipment. The standard is designed to minimize risks and ensure that medical devices can be used safely in the complex environments of healthcare facilities. It encompasses various aspects of medical device safety, including electrical, mechanical, and thermal factors.
Importance of Leakage Testing
Leakage testing is a critical component of the IEC 60601 standard, as it helps verify that medical devices meet safety requirements before they are approved for clinical use. The testing process involves measuring the leakage current in different scenarios to ensure that it remains within acceptable limits. This ensures that even in the case of a fault, the risk of electrical shock to the patient or operator is minimized.
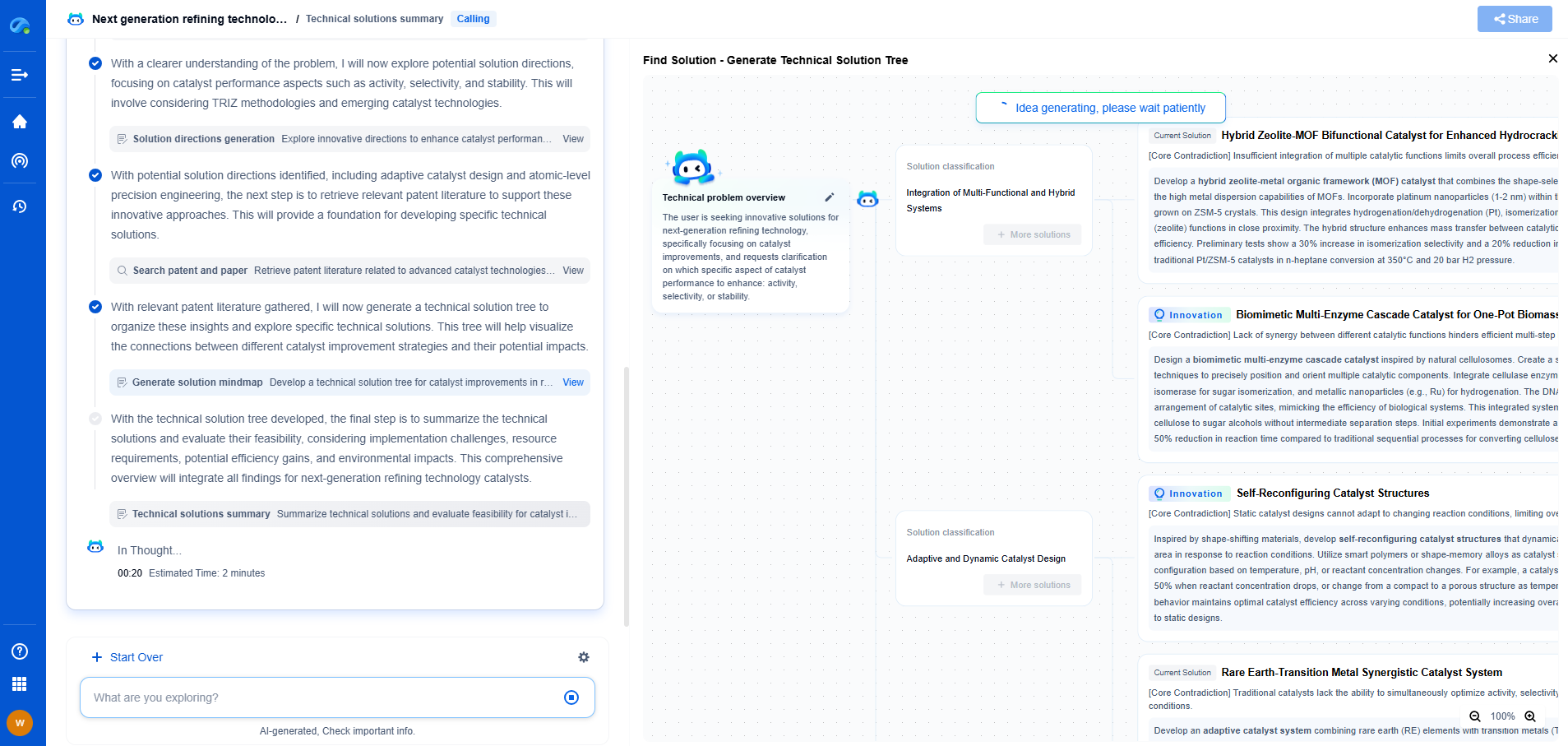
Types of Leakage Current Measurements
1. **Earth Leakage Current**: This current flows from the equipment's live parts through the protective grounding conductor. Testing for earth leakage current ensures that the device's grounding system is effective in preventing electrical shock in case of insulation failure.
2. **Enclosure Leakage Current**: This measures current that may flow from the device’s enclosure to ground or other conductive paths. Ensuring that enclosure leakage is within safe limits is vital for preventing accidental shocks through touch.
3. **Patient Leakage Current**: Perhaps the most critical measurement, patient leakage current assesses the current that could flow through a patient connected to the device. This test ensures safety even when the patient is in direct contact with the medical equipment.
4. **Patient Auxiliary Current**: This current flows between parts of the equipment that are not intended to be connected but could be during normal use. Testing for this ensures that any stray currents do not adversely affect the patient.
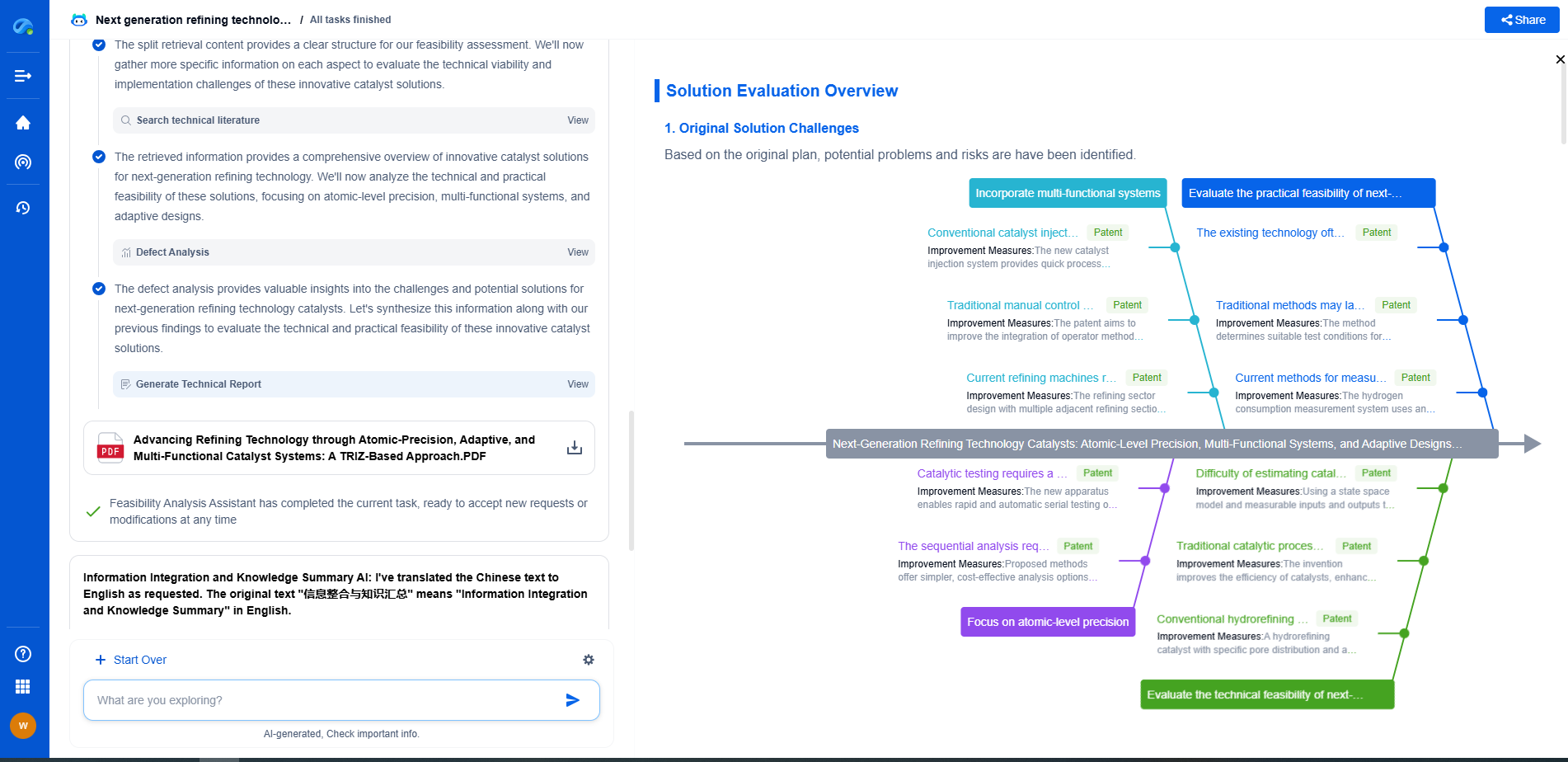
Testing Procedures and Equipment
To conduct leakage testing, specialized equipment such as leakage current testers or analyzers are used. These devices simulate different fault conditions and measure the resulting leakage currents. Testing is typically performed under normal conditions as well as under single-fault conditions to ensure comprehensive safety coverage. The results are then compared against the IEC 60601 standard limits to determine compliance.
Ensuring Compliance
Compliance with IEC 60601 is not just about passing a test; it involves a thorough design process that integrates safety from the ground up. Manufacturers need to ensure that all components, from power supply to chassis design, are optimized to minimize leakage currents. Regular updates and revisions to the standard mean that staying compliant is an ongoing process, requiring continuous attention to new safety insights and technological advancements.
The Impact on Medical Device Development
Adherence to IEC 60601 and its leakage testing requirements significantly impacts the development timeline and cost of medical devices. While rigorous, these standards drive innovation by encouraging the development of safer, more reliable products. Devices that meet these standards are more likely to gain regulatory approval and user trust, ultimately leading to broader market acceptance.
Conclusion
Medical equipment leakage testing is an essential part of ensuring the safety and efficacy of devices used in healthcare settings. By adhering to the IEC 60601 standards, manufacturers can significantly reduce the risk of electrical hazards, protecting both patients and healthcare providers. As technology evolves, so too will the standards, ensuring that safety remains at the forefront of medical device innovation.
Navigating the evolving world of electrical measurement—from high-precision signal integrity to advanced test protocols like BERT or TDR—demands more than just expertise; it demands smart tools.
Patsnap Eureka empowers you to keep up—by turning complex patent data, technical parameters, and industry signals into actionable insight. It’s your AI partner for exploring what’s next in test, measurement, and electrical diagnostics.
💡 Try Patsnap Eureka for free and see how it transforms the way you work with electrical measurement technologies.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com