Membrane Electrode Assembly (MEA): The Heart of Fuel Cell Technology
JUN 20, 2025 |
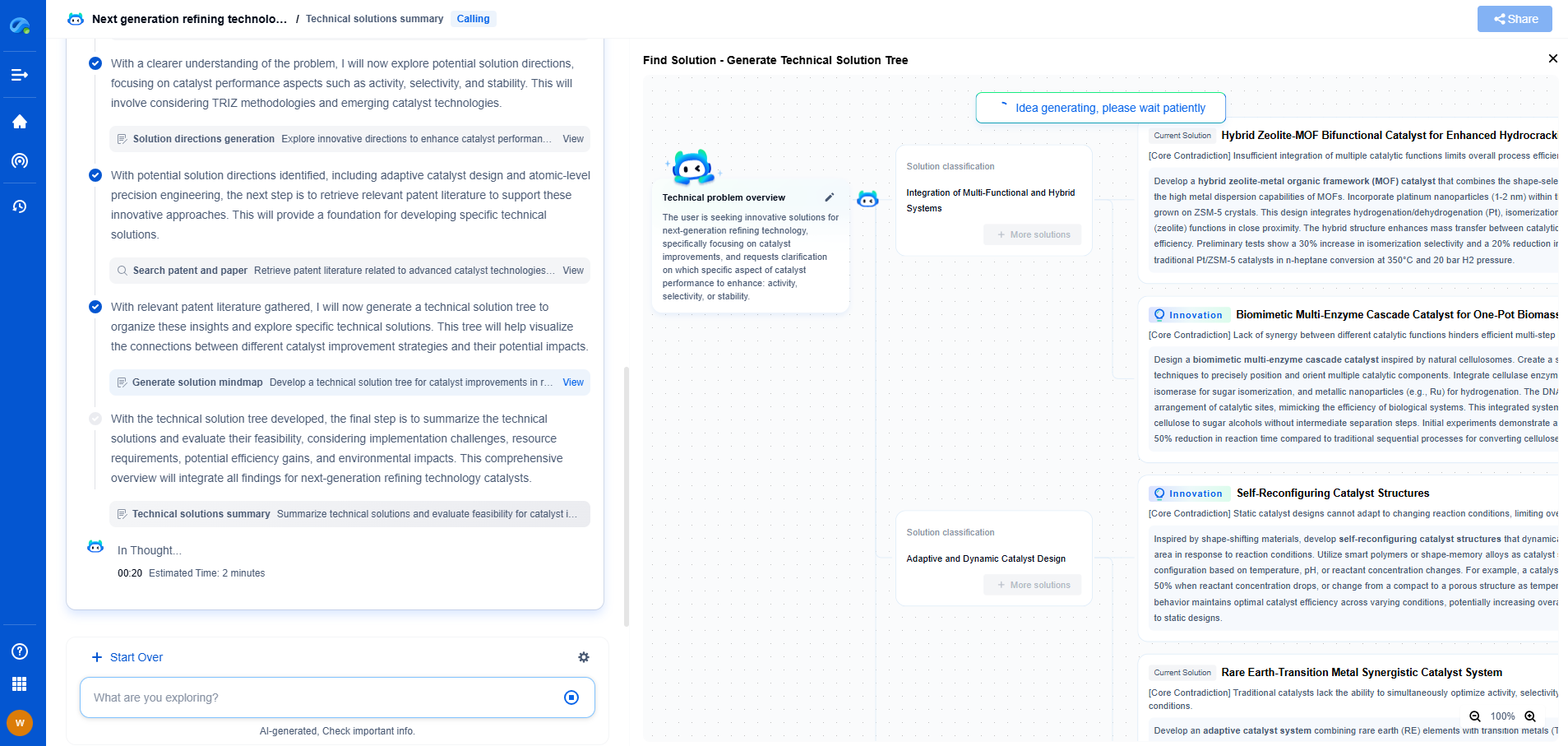
The Structure of a Membrane Electrode Assembly
The Membrane Electrode Assembly is comprised of several key components that work together to facilitate the electrochemical reactions necessary for energy conversion. These components typically include the proton exchange membrane (PEM), catalyst layers, gas diffusion layers, and sometimes a backing layer.
The proton exchange membrane is a crucial component that serves as an electrolyte, allowing protons to pass through while being impermeable to gases like hydrogen and oxygen. It effectively separates the anode and cathode sides of the fuel cell, preventing the mixing of reactant gases and ensuring efficient proton transfer. On either side of the membrane are the catalyst layers, commonly made from platinum-based materials, which facilitate the electrochemical reactions by lowering the activation energy required for the reactions to occur.
The gas diffusion layers, situated adjacent to the catalyst layers, are responsible for ensuring a uniform distribution of gases across the catalyst surface, facilitating efficient gas transport and water management within the cell. These layers often consist of porous carbon materials that provide pathways for gas flow, electron conduction, and water removal.
The Role of MEA in Fuel Cell Operation
The Membrane Electrode Assembly is integral to the operation of a fuel cell, as it is where the electrochemical reactions take place, converting hydrogen and oxygen into electricity and water. At the anode, hydrogen molecules are split into protons and electrons by the catalyst. The protons migrate through the proton exchange membrane to the cathode, while the electrons are forced to travel through an external circuit, generating an electric current.
At the cathode, oxygen molecules combine with the protons and electrons, facilitated by the catalyst, to produce water and heat. This sequence of reactions, enabled by the MEA, is what generates power in fuel cell systems, highlighting the importance of the assembly's design and materials in influencing the efficiency and durability of fuel cells.
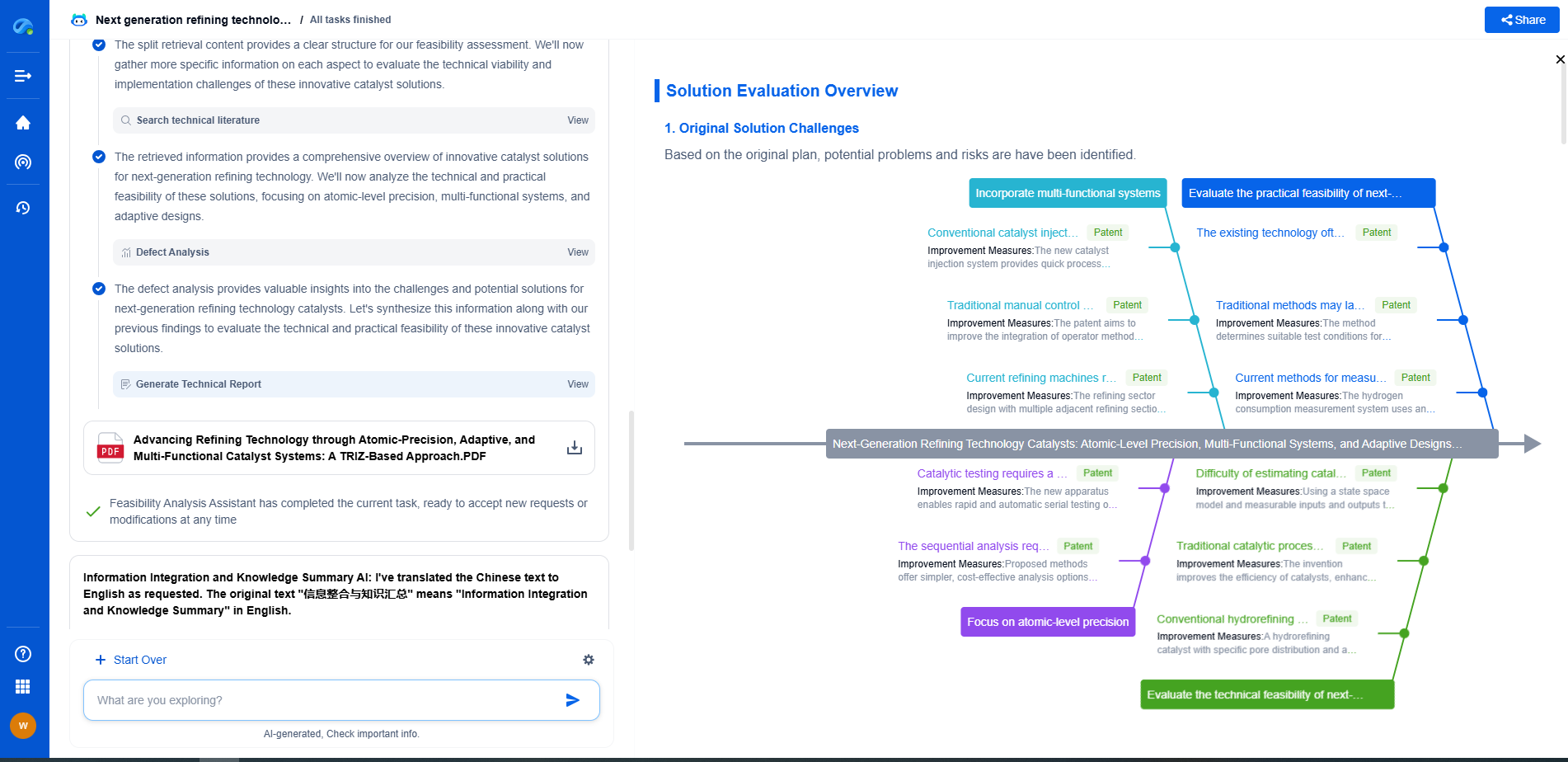
Challenges and Advancements in MEA Technology
Despite the critical role of the Membrane Electrode Assembly in fuel cell technology, several challenges must be addressed to optimize its performance and commercialization. One of the primary challenges is the cost of materials, particularly the platinum-based catalysts, which remain expensive and have limited availability. Researchers are actively exploring alternative materials and catalyst designs to reduce costs without compromising performance.
Additionally, the durability and longevity of MEAs are crucial for the widespread adoption of fuel cells. Degradation of MEA components, such as membrane thinning and catalyst deactivation, can significantly impact fuel cell lifespan. Advancements in materials science, such as the development of more robust membranes and improved catalyst formulations, are essential for enhancing the durability of MEAs.
Emerging technologies, such as nanostructured catalyst materials and innovative membrane designs, are being explored to address these challenges. These advancements hold the promise of making fuel cells more cost-effective and reliable, paving the way for broader applications in transportation, stationary power generation, and portable devices.
Conclusion
The Membrane Electrode Assembly is undeniably the heart of fuel cell technology, playing a pivotal role in the conversion of chemical energy to electrical energy. Its intricate structure and function are fundamental to the efficiency and performance of fuel cells. As research and development continue to advance, overcoming the challenges associated with MEA technology will be essential for unlocking the full potential of fuel cells as a clean and sustainable energy source. Understanding and improving MEA components will be key to the future of fuel cell technology, driving innovations that will shape the energy landscape for years to come.
Accelerate Breakthroughs in Fuel Cell and Battery Innovation—with the Power of AI
From solid-state battery breakthroughs to high-efficiency hydrogen fuel cells, keeping pace with fast-evolving chemistries, global patent landscapes, and emerging application pathways is an ever-growing challenge for R&D and IP professionals.
Patsnap Eureka, our intelligent AI assistant built for R&D professionals in high-tech sectors, empowers you with real-time expert-level analysis, technology roadmap exploration, and strategic mapping of core patents—all within a seamless, user-friendly interface.
Whether you're optimizing cathode formulations, evaluating electrolyte stability, or navigating the crowded patent space around battery pack design, Eureka empowers you to move faster and with greater confidence.
Start your journey with Patsnap Eureka today—streamline your research, enhance decision-making, and power the future of energy with AI-driven clarity.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com