Optical Emission Spectroscopy (OES) Setup: Identifying Spectral Lines for Argon vs. Nitrogen Plasmas
JUN 26, 2025 |
Optical Emission Spectroscopy (OES) is a vital analytical technique used to study the light emitted by excited atoms or molecules. When a sample is energized, the atoms or molecules transition to higher energy states. As they return to their ground state, they emit light at specific wavelengths. This emission forms a spectrum, which can be analyzed to identify the elements present in the sample. OES is extensively employed in plasma diagnostics due to its non-invasive nature and capability to provide real-time analysis.
Key Differences Between Argon and Nitrogen Plasmas
Argon and nitrogen are two commonly used gases in plasma processes, each having distinct properties that influence their spectral lines. Argon, an inert gas, typically shows sharp, well-defined spectral lines, making it easier to detect and analyze. In contrast, nitrogen, being a diatomic molecule, has more complex spectra due to additional molecular transitions, resulting in broader spectral features and a range of vibrational and rotational lines.
Setting Up an Optical Emission Spectroscopy System
Setting up an OES system requires careful consideration of several components: a plasma source, a spectrometer, and a detector. The plasma source generates the excited atoms or molecules. Plasma can be created using various devices such as inductively coupled plasma or microwave plasma systems. The spectrometer disperses the emitted light into its component wavelengths, and the detector records the intensity of the light at each wavelength.
Calibration and Instrumentation
Calibration is a crucial step in ensuring accurate spectral line identification. The spectrometer needs to be calibrated using known reference lines, often from a mercury or neon lamp. The detector, which could be a photomultiplier tube or a charge-coupled device (CCD), must be sensitive enough to detect faint emissions and have a wide dynamic range to capture the varying intensities of different spectral lines.
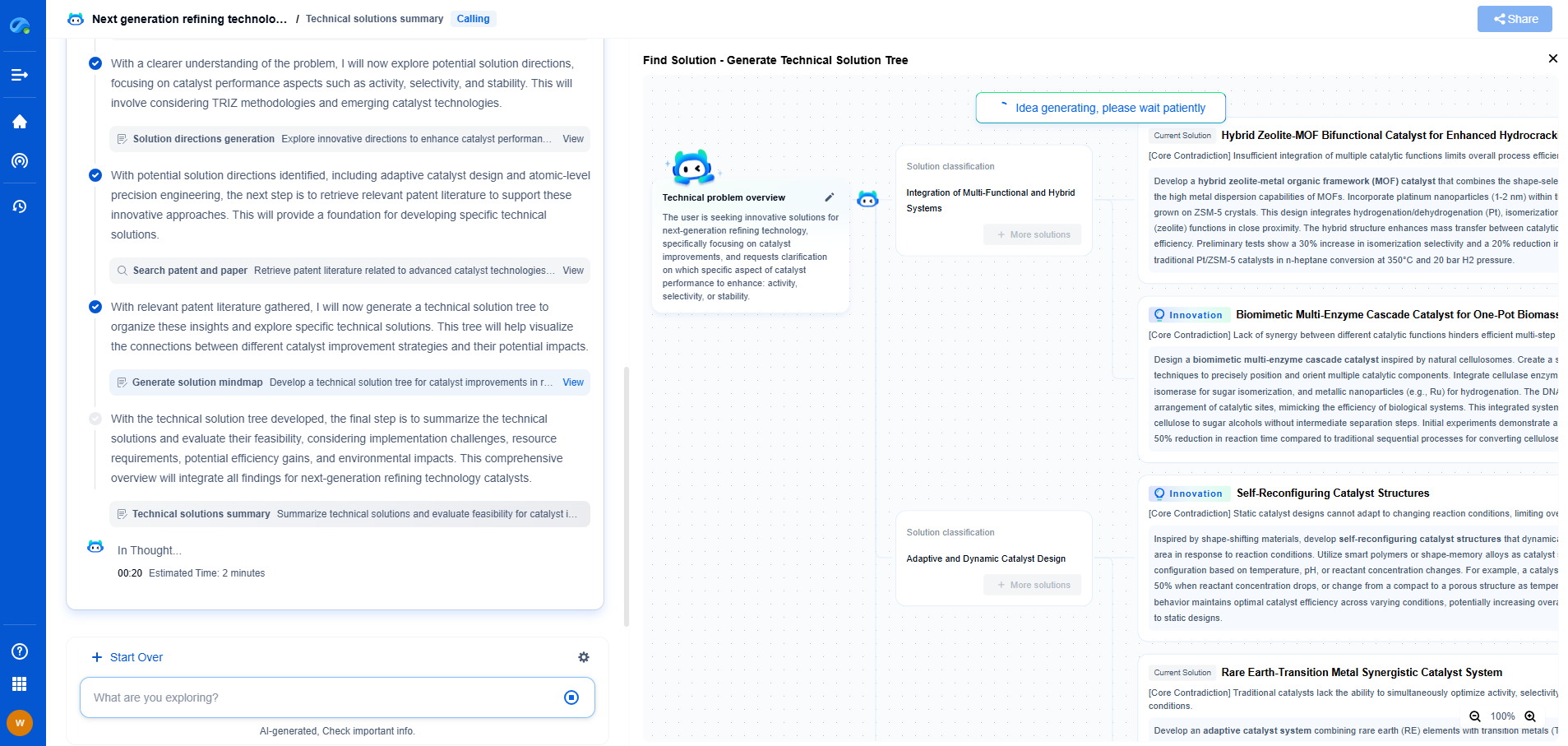
Identifying Spectral Lines for Argon Plasmas
In argon plasmas, the identification of spectral lines is relatively straightforward due to the noble gas’s simple electronic structure. Argon emission lines are typically found in the visible to near-infrared region. The most prominent lines are the argon ion (Ar II) and neutral argon (Ar I) lines, with strong emissions around 696.5 nm, 706.7 nm, and 772.4 nm. These lines serve as key indicators in monitoring and characterizing plasma processes.
Identifying Spectral Lines for Nitrogen Plasmas
Nitrogen plasmas present a more complex spectral analysis challenge. Due to its molecular nature, nitrogen emits spectra that include molecular nitrogen (N2) and nitrogen ions (N2+), leading to bands rather than discrete lines. The most significant emissions occur in the ultraviolet and visible regions, with the Second Positive System and the First Negative System being highly prominent. These systems are associated with transitions in molecular nitrogen and its ions, typically observed around 337.1 nm, 357.7 nm, and 391.4 nm.
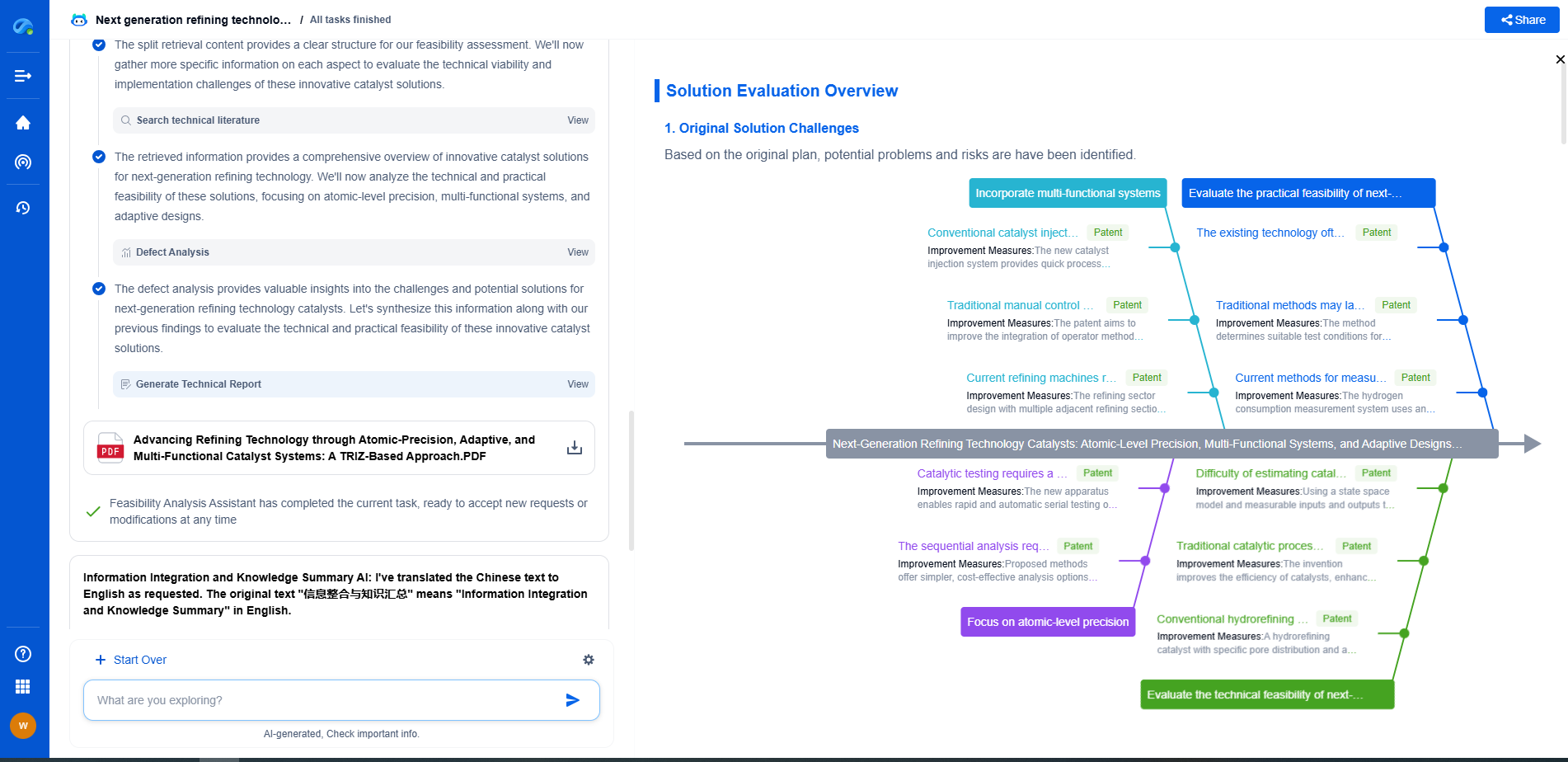
Challenges in Discriminating Between Argon and Nitrogen Spectra
Discriminating between argon and nitrogen spectral lines can be challenging, especially in mixed-gas plasmas. Overlapping spectral lines and varying intensities require sophisticated data analysis techniques. Software tools can aid in deconvoluting complex spectra and resolving overlapping peaks to accurately identify the contributions from each gas.
Applications of OES in Plasma Diagnostics
OES is invaluable in various applications, from semiconductor manufacturing to environmental monitoring. In semiconductor processing, OES allows for the real-time control of plasma etching and deposition processes, ensuring precision and uniformity. In environmental science, OES helps in monitoring air quality by detecting trace gases and pollutants.
Conclusion
Optical Emission Spectroscopy is a powerful tool for identifying and analyzing the spectral lines of gases like argon and nitrogen in plasma applications. While argon provides clear and distinct spectral lines, nitrogen's more complex spectra require detailed analysis. Understanding these differences and effectively setting up an OES system enables precise diagnostics and enhances the efficiency of plasma-related processes. This knowledge is crucial for advancing technologies across various industries, ensuring quality and innovation in plasma applications.
Empower Electromagnetic Innovation with Patsnap Eureka
From high-frequency antenna arrays and electromagnetic shielding to plasma propulsion and wave-based energy transfer, the electromagnetic domain sits at the core of next-generation technologies. Yet navigating its vast landscape of patents, research papers, and evolving technical standards can be time-consuming and complex.
Patsnap Eureka, our intelligent AI assistant built for R&D professionals in high-tech sectors, empowers you with real-time expert-level analysis, technology roadmap exploration, and strategic mapping of core patents—all within a seamless, user-friendly interface.
👉 Experience Patsnap Eureka today and transform how your team navigates the complexity of electromagnetic innovation.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com