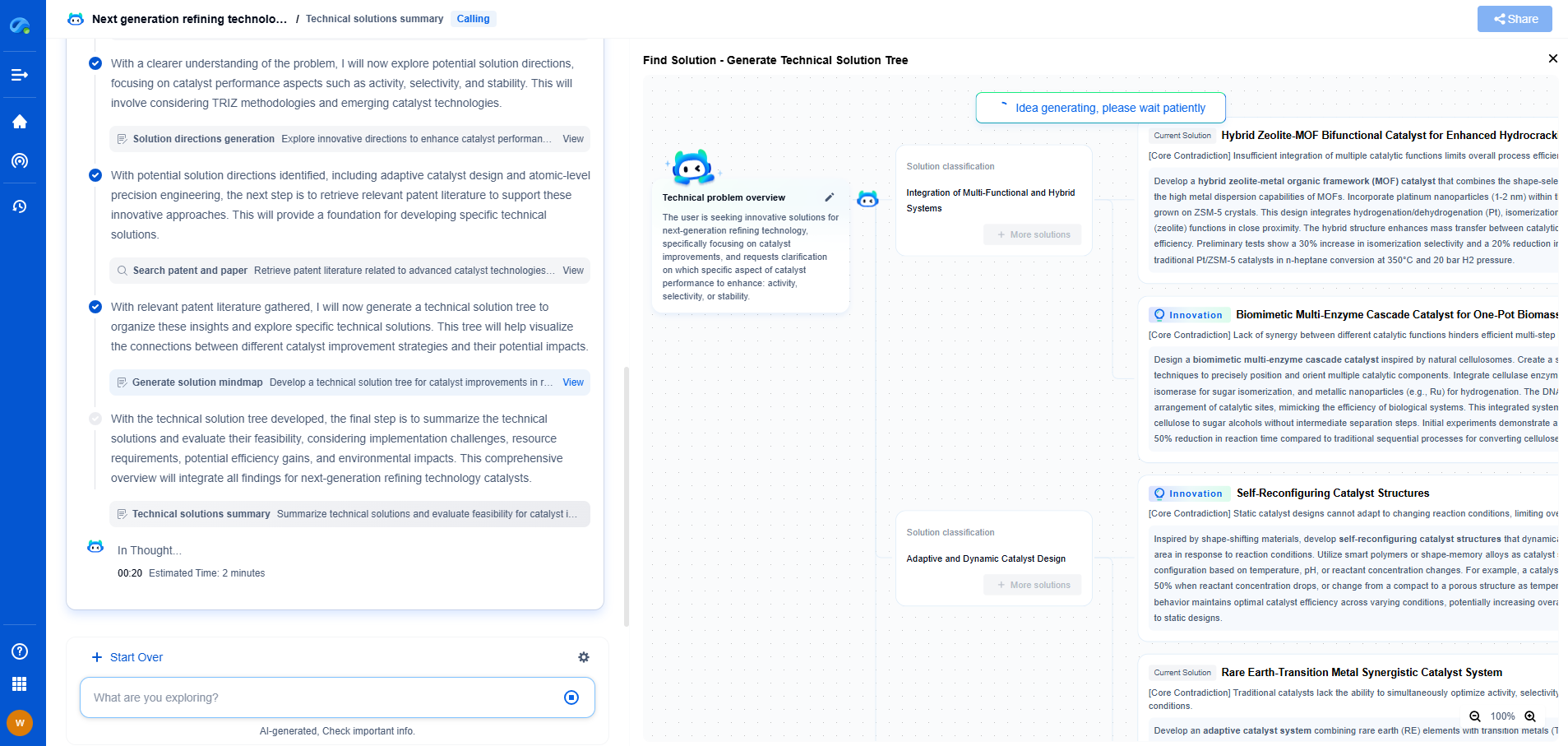
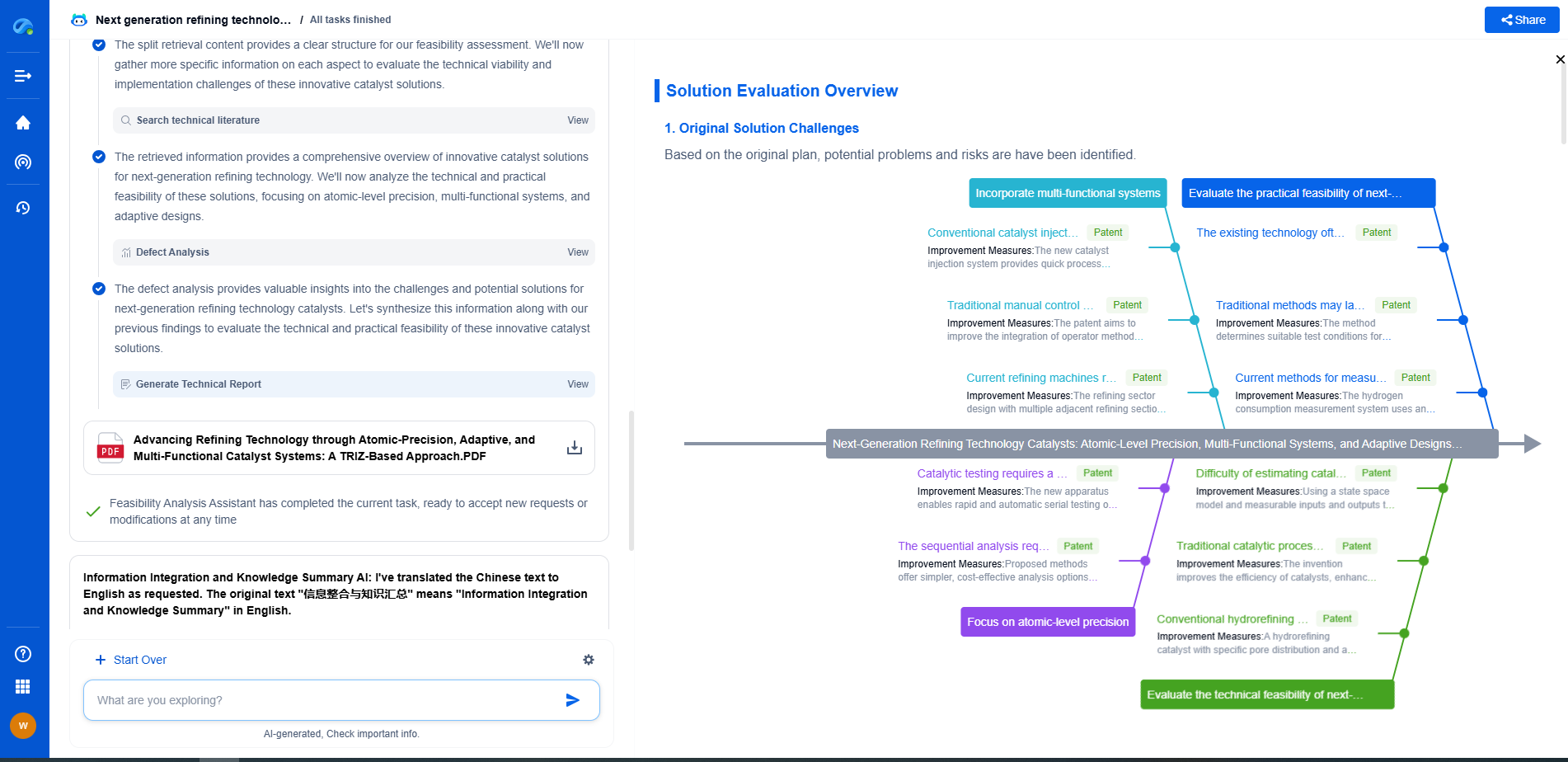
Pharmaceutical QA: USP-Compliant UV-Vis Drug Purity Testing
JUL 15, 2025 |
The pharmaceutical industry continually strives to maintain the highest standards of quality and safety. Central to this effort is the rigorous testing of drug purity. Among the various analytical techniques available, UV-Vis spectroscopy has emerged as a reliable and efficient method, particularly when aligned with the standards set by the United States Pharmacopeia (USP). In this blog, we will delve into the essentials of UV-Vis drug purity testing, exploring its principles, advantages, and its role in ensuring compliance with USP guidelines.
Understanding UV-Vis Spectroscopy
UV-Vis spectroscopy involves the absorption of ultraviolet or visible light by a substance, which provides valuable information about its purity and concentration. The technique is based on the principle that molecules absorb light at specific wavelengths, which can be measured to determine the presence and concentration of particular compounds. In the pharmaceutical context, this method is instrumental in assessing the purity of active pharmaceutical ingredients (APIs) and finished products.
USP Guidelines for UV-Vis Testing
The USP sets stringent guidelines to ensure that pharmaceutical products meet quality standards. For UV-Vis spectroscopy, the USP provides specific protocols that must be adhered to in order to guarantee accurate and reproducible results. These guidelines cover aspects such as instrument calibration, validation of methods, sample preparation, and data interpretation. By following these standards, pharmaceutical companies can ensure that their products are free from impurities and safe for consumer use.
Advantages of UV-Vis Spectroscopy in Drug Purity Testing
UV-Vis spectroscopy offers several advantages as a method for drug purity testing. These include:
1. **Non-Destructive Analysis**: One of the primary benefits of UV-Vis spectroscopy is that it is a non-destructive method. This means that the sample remains intact and can be subjected to further testing if necessary.
2. **Rapid and Cost-Effective**: UV-Vis spectroscopy provides quick results, making it an efficient method for routine quality control. Additionally, it is relatively low-cost compared to other analytical techniques, making it accessible for regular use.
3. **High Sensitivity and Accuracy**: With proper calibration and validation, UV-Vis spectroscopy can achieve high sensitivity and accuracy, allowing for the detection of even trace levels of impurities.
4. **Wide Range of Applications**: This technique is versatile and can be used for a variety of pharmaceutical products, from raw materials to finished dosage forms.
Implementing USP-Compliant UV-Vis Testing
To implement USP-compliant UV-Vis testing, pharmaceutical companies must first ensure that their spectrophotometers are properly calibrated and maintained. Regular calibration checks using standard reference materials are essential to maintain the accuracy and reliability of the equipment.
Moreover, method validation is a critical component of compliance. This involves demonstrating that the analytical method is suitable for its intended purpose, which includes evaluating parameters such as specificity, linearity, accuracy, precision, and limit of detection. Proper documentation of the validation process is crucial to demonstrate compliance with USP standards.
Sample preparation is another important consideration. The USP guidelines provide detailed instructions on how to prepare samples to ensure consistent and accurate measurements. This often involves dissolving the sample in a suitable solvent, filtering it to remove particulates, and ensuring that the concentration is within the linear range of the spectrophotometer.
Challenges and Considerations
While UV-Vis spectroscopy is a powerful tool for drug purity testing, it is not without its challenges. One of the main considerations is the potential for interference from other substances present in the sample. This requires careful selection of wavelengths and, in some cases, the use of additional techniques to isolate the compound of interest.
Another challenge is ensuring that the method remains robust and reliable over time. This requires ongoing training for laboratory personnel and regular review of methods and procedures to keep up with advances in technology and changes in regulatory requirements.
Conclusion: Ensuring Quality and Compliance
In conclusion, UV-Vis spectroscopy, when conducted in compliance with USP guidelines, is an invaluable tool for ensuring the purity and quality of pharmaceutical products. By adhering to these standards, companies can not only comply with regulatory requirements but also protect consumer safety and maintain the integrity of their products. As the industry continues to evolve, embracing accurate and efficient testing methods like UV-Vis spectroscopy will remain crucial in the ongoing quest for excellence in pharmaceutical quality assurance.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com