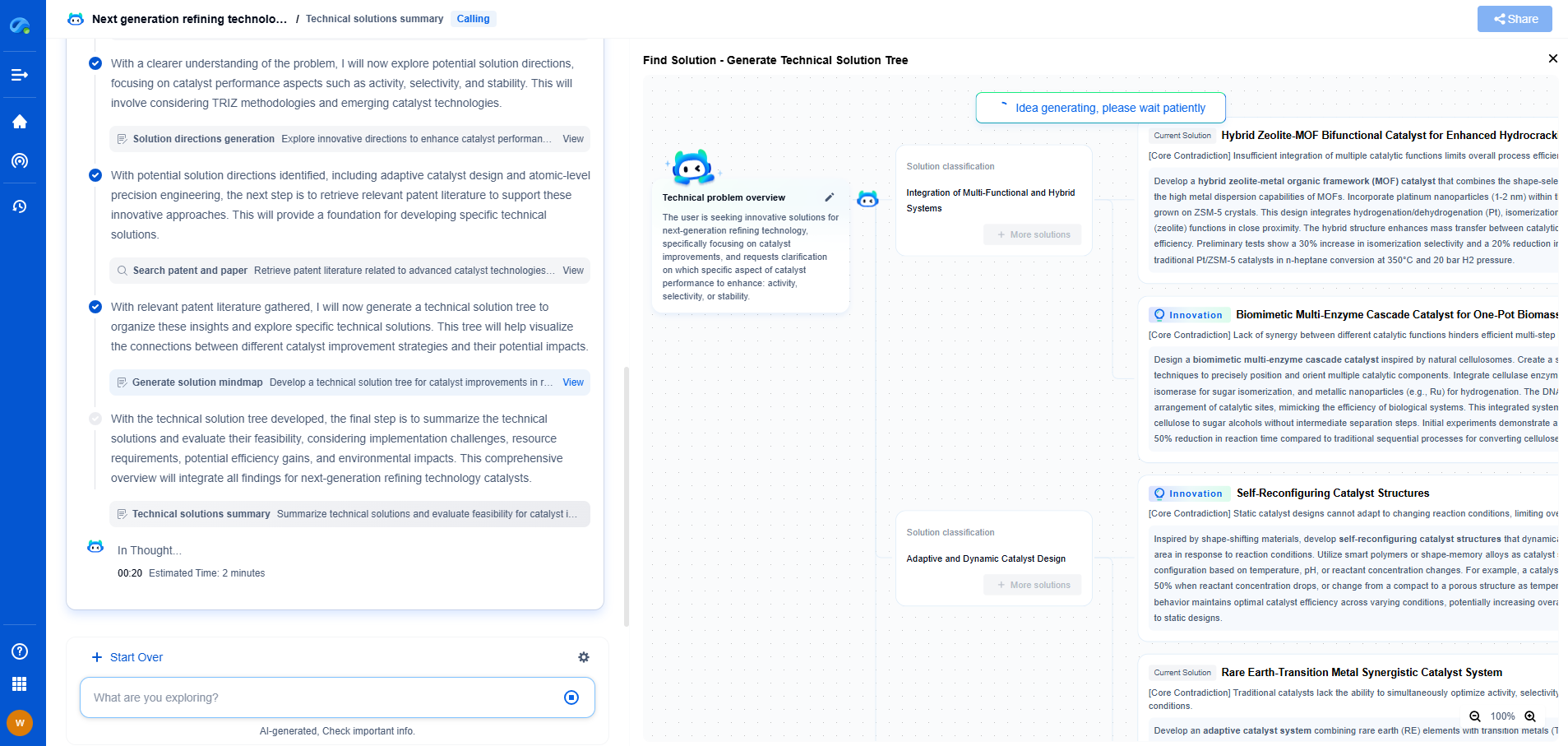
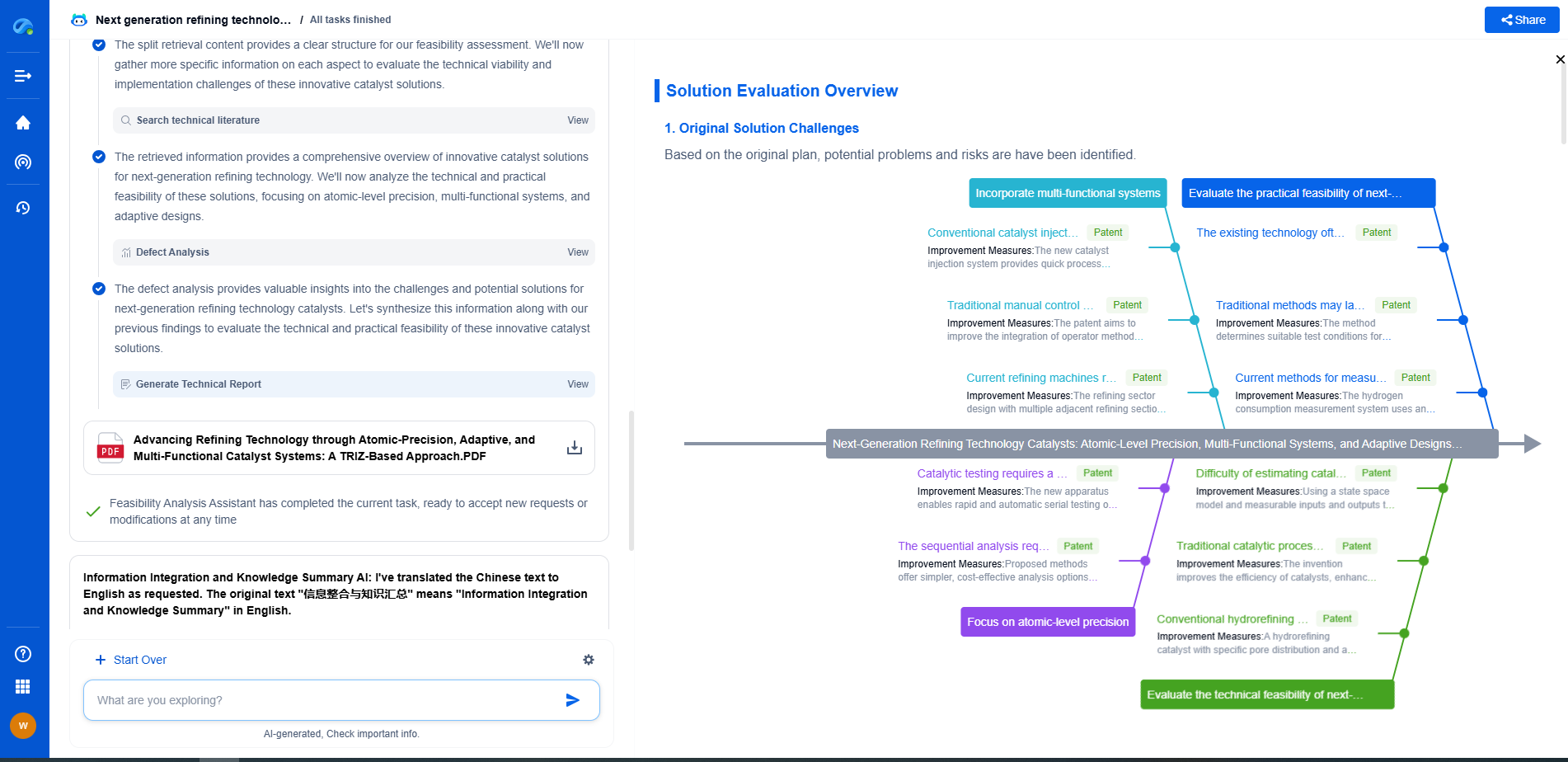
Raman Spectroscopy vs Infrared Absorption: Key Differences and Use Cases
JUL 15, 2025 |
Spectroscopy is a powerful analytical tool used in various scientific fields to study the interaction between matter and electromagnetic radiation. Among the diverse types of spectroscopy, Raman spectroscopy and infrared (IR) absorption spectroscopy are two prominent methods frequently employed to determine molecular composition and structure. Both techniques offer unique advantages and are suited for different applications. This article aims to highlight the key differences between Raman spectroscopy and infrared absorption, as well as their specific use cases.
Fundamental Principles
Raman Spectroscopy:
Raman spectroscopy is based on the inelastic scattering of photons, known as Raman scattering. When light interacts with a molecule, most photons are scattered elastically (Rayleigh scattering), but a small fraction are scattered inelastically, resulting in a shift in energy corresponding to molecular vibrations. This shift provides a molecular fingerprint that can be used for identification and analysis.
Infrared Absorption Spectroscopy:
Infrared absorption spectroscopy, on the other hand, involves the absorption of infrared light by molecules, causing transitions between vibrational energy levels. The absorbed frequencies correspond to different molecular vibrations, providing a spectrum that can be used to identify functional groups and molecular structures.
Key Differences
Mechanism of Interaction:
The fundamental difference between the two techniques lies in their interaction mechanisms. Raman spectroscopy relies on scattering, while infrared spectroscopy is based on absorption. This distinction affects how each technique can be applied and the type of information they provide.
Instrumentation:
Raman spectroscopy typically requires a laser light source and a sensitive detector to measure the scattered light. Infrared spectroscopy uses an infrared light source and an infrared detector to measure absorbed light. These differences in instrumentation can influence factors such as cost, setup complexity, and the convenience of use.
Sensitivity to Molecular Symmetry:
One of the notable differences is their sensitivity to molecular symmetry. Raman spectroscopy is more sensitive to non-polar bonds and symmetric molecules, while infrared spectroscopy is more sensitive to polar bonds and asymmetric molecules. This makes Raman spectroscopy advantageous for analyzing inorganic compounds and conjugated systems, whereas infrared spectroscopy is well-suited for organic compounds with distinct functional groups.
Sample Preparation:
Raman spectroscopy generally requires minimal sample preparation and can analyze samples in various states, including solids, liquids, and gases. Infrared spectroscopy often requires more extensive sample preparation, especially for solid samples, which may need to be ground into a fine powder or pressed into pellets.
Complementary Nature
Despite their differences, Raman and infrared spectroscopy are often used in a complementary manner. The information obtained from one technique can enhance the understanding of results obtained from the other. For instance, a molecule that exhibits weak Raman scattering but strong infrared absorption can be studied more comprehensively by combining both techniques. This complementary use is particularly valuable in complex mixtures or when dealing with intricate molecular structures.
Use Cases
Raman Spectroscopy Applications:
Raman spectroscopy is widely used in fields such as chemistry, materials science, and biology. It is particularly useful for studying inorganic compounds, carbon materials like graphene, and biological samples such as cells and tissues. Its ability to provide detailed information with minimal sample preparation makes it ideal for forensic analysis and quality control in pharmaceuticals.
Infrared Absorption Spectroscopy Applications:
Infrared spectroscopy is extensively utilized in organic chemistry for functional group identification and molecular structure determination. It plays a crucial role in the analysis of polymers, pharmaceuticals, and environmental samples. The technique's sensitivity to polar bonds makes it invaluable for studying organic compounds and biological molecules like proteins and lipids.
Conclusion
In summary, Raman spectroscopy and infrared absorption spectroscopy are distinct yet complementary techniques that offer unique insights into molecular composition and structure. Understanding their differences and specific advantages allows scientists and researchers to choose the appropriate method for their analytical needs. Whether in academic research, industrial applications, or forensic investigations, both techniques continue to play a crucial role in advancing our understanding of the molecular world.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com