What Is Fluorometry? Principles and Instrumentation Explained
JUL 15, 2025 |
Understanding Fluorometry
Fluorometry, also known as fluorescence spectroscopy, is a method that measures the intensity of fluorescent light emitted by a substance. When a material absorbs light or electromagnetic radiation, it can become excited; fluorometry specifically examines substances that emit light as they return to their ground state from this excited state. The emitted light is usually at a longer wavelength than the absorbed light due to the loss of energy through various non-radiative pathways. The difference between the absorbed and emitted wavelengths is known as the Stokes shift.
Principles of Fluorescence
At the heart of fluorometry is the principle of fluorescence, a photophysical process whereby a molecule absorbs photons and re-emits them as light. The key processes involved in fluorescence include excitation, where a molecule absorbs a photon and transitions to a higher energy state, and emission, where the molecule returns to its original state, releasing a photon in the process. The intensity of the emitted light is proportional to the concentration of the fluorescent molecules in the sample, which makes fluorometry a quantitative technique.
Factors Influencing Fluorescence
Several factors influence the fluorescence of a substance, including the nature of the fluorescent molecule, the environment (such as pH and temperature), and the presence of quenching agents. Quenching refers to any process that decreases the fluorescence intensity of a substance, such as collisional quenching or self-quenching. Understanding these factors is crucial for accurate measurements and interpretations in fluorometry.
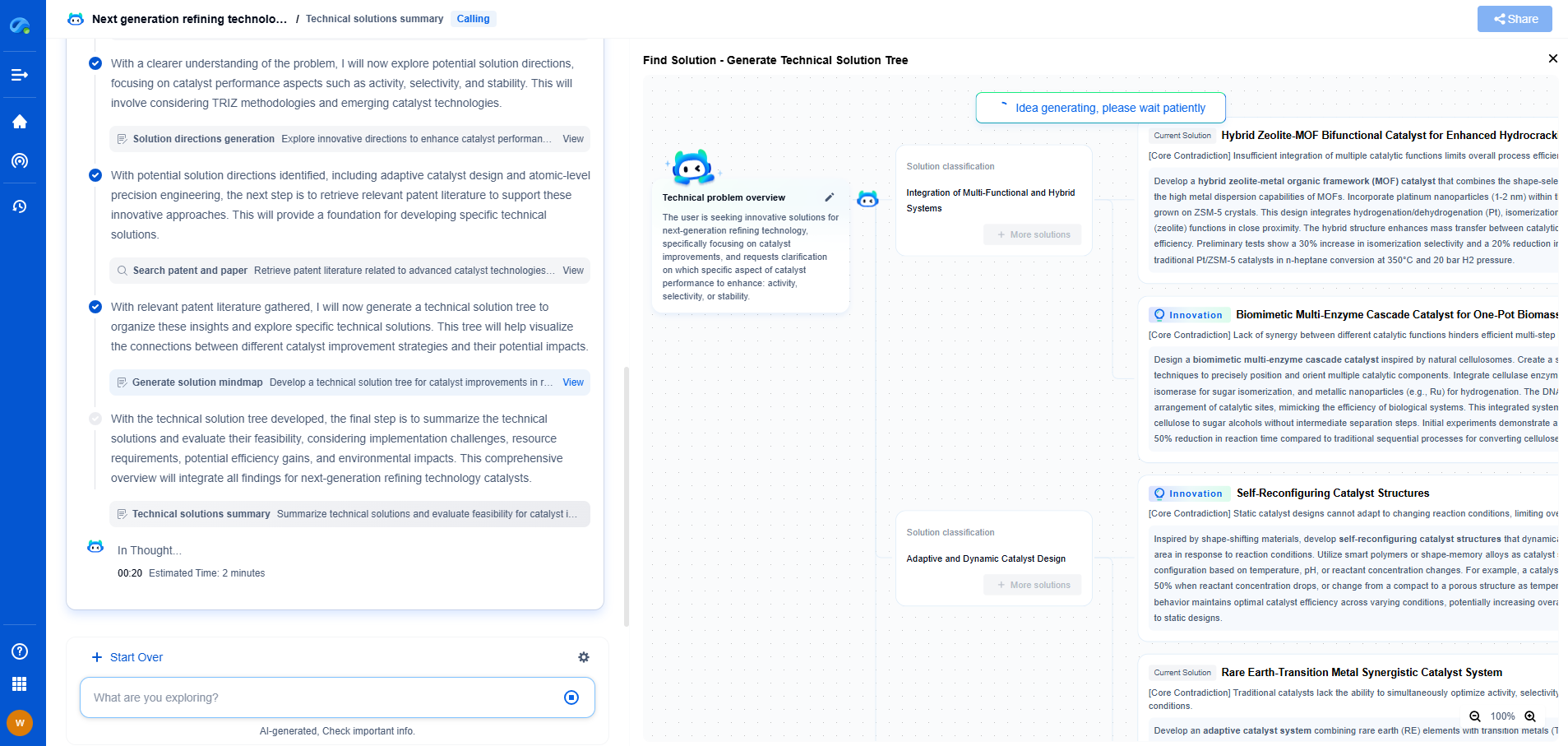
Fluorometry Instrumentation
A typical fluorometer consists of several key components: a light source, a monochromator, a sample holder, and a detector.
1. Light Source: The light source provides the excitation energy needed to excite the fluorescent molecules. Common light sources include xenon lamps, mercury lamps, and LEDs. The choice of light source depends on the required intensity and stability.
2. Monochromator: This component selects the specific wavelength of light used to excite the sample. It also helps isolate the emission wavelength for detection, ensuring that only the fluorescent light is measured. Monochromators are pivotal in differentiating between excitation and emission spectra, which aids in accurate analysis.
3. Sample Holder: The sample holder, often a cuvette or a microplate, is where the sample is placed for measurement. It must be made of a material that does not interfere with the fluorescence measurement, such as quartz or certain plastics.
4. Detector: The detector captures the emitted light and converts it into an electrical signal. Photomultiplier tubes (PMTs) and charge-coupled devices (CCDs) are commonly used detectors in fluorometry. The detector’s sensitivity and dynamic range are critical for measuring low concentrations of fluorescent molecules.
Applications of Fluorometry
Fluorometry finds application in a diverse range of fields, from medical diagnostics to environmental monitoring. In biochemistry, it is employed for the quantification of nucleic acids and proteins, often using fluorescent dyes. In clinical diagnostics, fluorometry aids in the detection of biomarkers and pathogens. Environmental scientists use fluorometry to monitor water quality by detecting pollutants like oil and heavy metals. Its applications extend further into areas such as food safety, pharmaceuticals, and chemical analysis.
Advantages and Limitations
Fluorometry offers several advantages, including high sensitivity, specificity, and the ability to conduct real-time analysis. However, it also has limitations, such as susceptibility to quenching and interference from background fluorescence. Proper calibration and sample preparation are essential to minimize these issues and ensure accurate results.
Conclusion
Fluorometry is a versatile and powerful technique that continues to play a critical role in scientific research and industry. By understanding its principles and mastering its instrumentation, researchers can harness its potential to uncover insights and solve complex analytical challenges. Whether it's in a laboratory setting or applied fieldwork, fluorometry remains an invaluable tool in the modern scientific arsenal.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com