What is Spectrophotometry and How Does It Work?
JUL 15, 2025 |
Spectrophotometry is a powerful analytical technique used to measure the intensity of light absorbed by a sample material. This tool is fundamental in fields such as chemistry, biology, and environmental science, providing valuable insights into the composition and concentration of various substances. By understanding the principles and applications of spectrophotometry, scientists can unravel complex chemical structures and monitor environmental changes with precision.
How Spectrophotometry Works
At its core, spectrophotometry is based on the principle of light absorption. When light passes through a sample, certain wavelengths are absorbed by the molecules in the material. The amount of light absorbed at each wavelength can be measured and used to identify and quantify different substances within the sample.
Components of a Spectrophotometer
A typical spectrophotometer consists of several key components: a light source, a monochromator, a sample holder, a detector, and a readout device. The light source emits a broad spectrum of light, often covering ultraviolet, visible, and infrared regions. The monochromator isolates specific wavelengths of light, directing them through the sample. The detector then measures the intensity of light that passes through or is reflected by the sample, while the readout device displays the results.
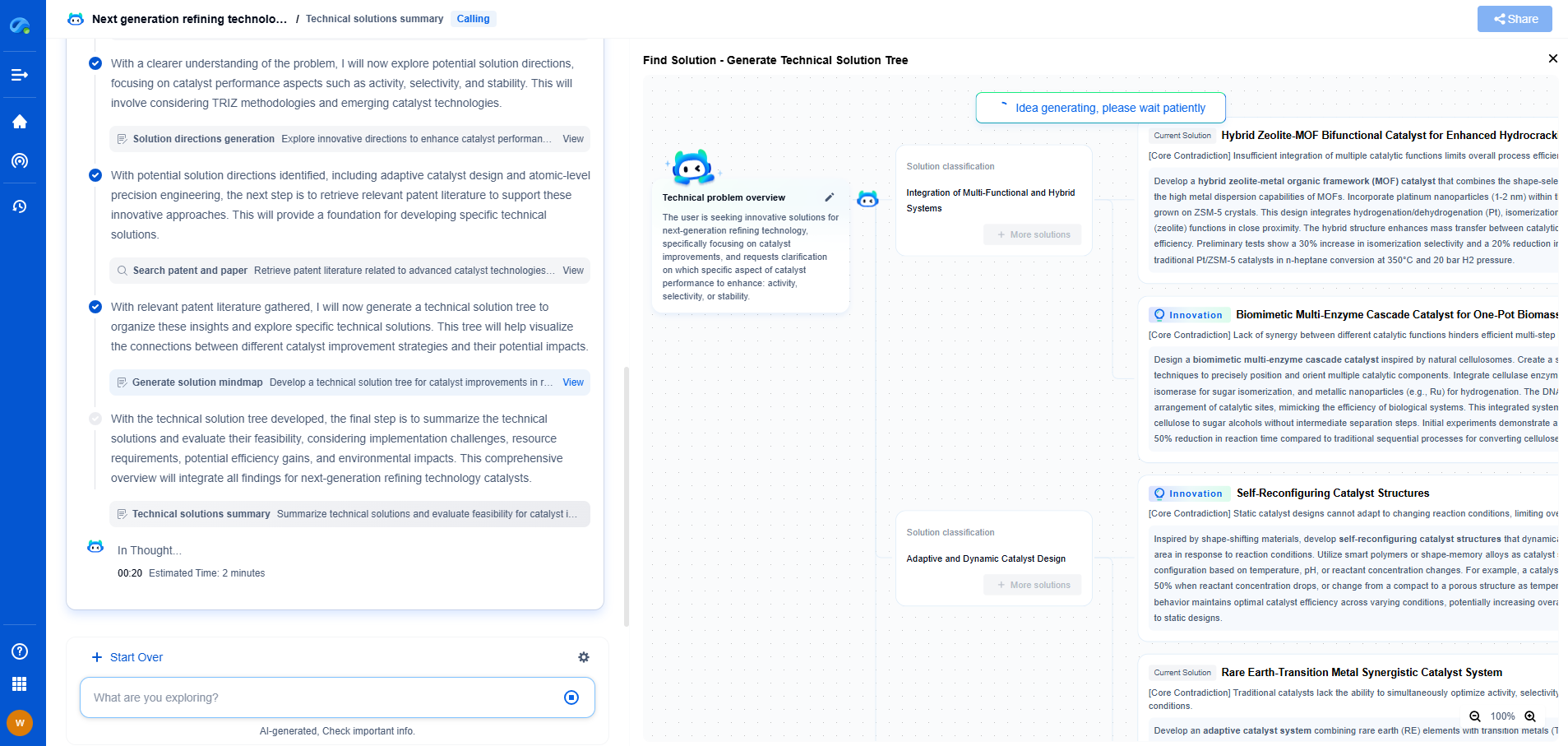
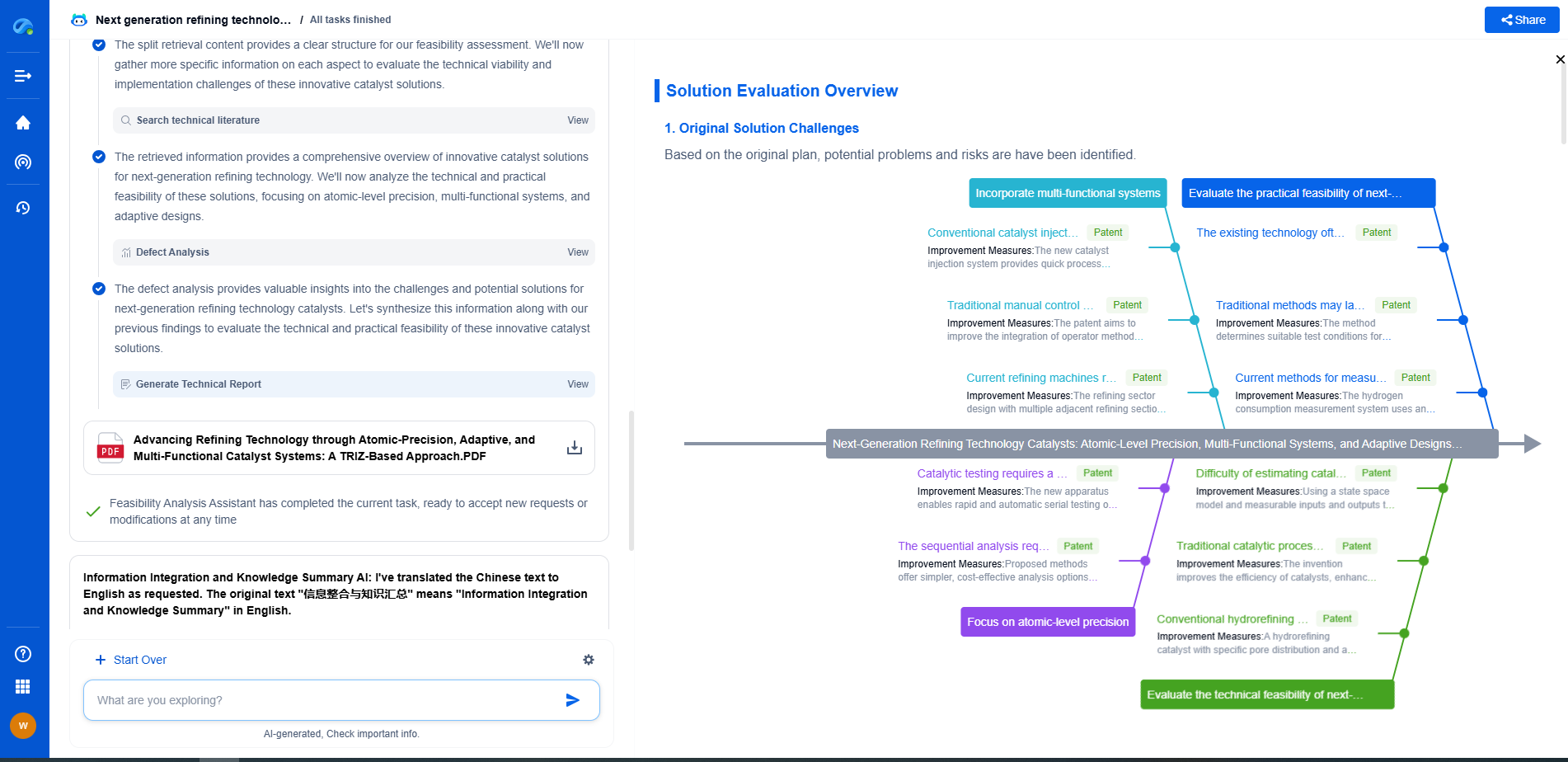
The Process of Spectrophotometric Analysis
The spectrophotometric analysis begins with calibrating the instrument using a blank sample, which contains only the solvent used to dissolve the sample material. This step accounts for any absorption by the solvent itself. The sample material is then placed in the sample holder, and light of a specific wavelength is directed through it. The detector measures the intensity of the transmitted light, and the difference between the initial and transmitted light intensities is used to calculate the sample’s absorbance.
The Beer-Lambert Law
The relationship between absorbance and concentration is expressed by the Beer-Lambert Law, which states that absorbance is directly proportional to the concentration of the absorbing species and the path length of the sample cell. This allows for the quantitative analysis of substances in a solution by comparing the absorbance values to those of standard solutions of known concentrations.
Applications of Spectrophotometry
Spectrophotometry has a wide range of applications in various scientific fields. In biochemistry, it is used to measure the concentration of nucleic acids and proteins, assess enzyme activities, and study reaction kinetics. Environmental scientists employ spectrophotometry to monitor pollutants in air and water, track changes in plant pigments, and analyze soil compositions. In the pharmaceutical industry, spectrophotometry is used for quality control and the development of new drugs.
Advantages and Limitations
The advantages of spectrophotometry include its accuracy, sensitivity, and versatility. It allows for rapid and non-destructive analysis of samples, making it an essential tool in many laboratories. However, spectrophotometry also has limitations. It requires clear and homogeneous samples, as turbidity or particulate matter can interfere with the measurements. Additionally, overlapping absorption peaks can complicate the analysis of complex mixtures.
Conclusion
Spectrophotometry is a vital technique in analytical science, offering detailed information about the composition and concentration of substances. Its application across various fields underscores its importance in both research and industry. By mastering the principles of spectrophotometry, scientists can continue to advance our understanding of the natural world and develop innovative solutions to global challenges.
From interferometers and spectroradiometers to laser displacement sensors and fiber optic probes, the field of optical measurement is evolving at light speed—driven by innovations in photonics, MEMS integration, and AI-enhanced signal processing.
With Patsnap Eureka, biomedical innovators can navigate cross-domain insights in optics, electronics, and biocompatible materials, while discovering IP trends across academic, clinical, and commercial datasets.
💡 Fuel your next breakthrough in optical health tech—start using Patsnap Eureka to unlock deep insights today.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com