Abstract
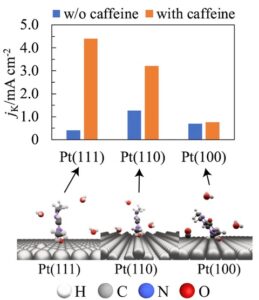
Enhancing the activity of the oxygen reduction reaction (ORR) is crucial for fuel cell development, and hydrophobic species are known to increase the ORR activity. This paper reports that caffeine enhanced the specific ORR activity of Pt(111) 11-fold compared to that without caffeine in a 0.1 M HClO4 aqueous solution. Moreover, caffeine increased the ORR activity of Pt(110) 2.5-fold; however, the activity of Pt(100) was unaffected. The infrared (IR) band of PtOH (blocking species of the ORR) decreased for all the surfaces. Caffeine was adsorbed with its molecular plane perpendicular to the Pt(111) and Pt(110) surfaces and tilted relative to the Pt(100) surface. Thus, the effects of caffeine on the ORR activity can be rationalized by a decrease in PtOH coverage and the difference in adsorption geometry of caffeine.
Introduction
A fuel cell is a power generation system with a higher energy conversion efficiency than that of thermal power generation. A major drawback of fuel cells is the high overpotential of the oxygen reduction reaction (ORR) at the air electrode, which causes energy loss in the system. Based on the high ORR overpotential, electrocatalysts in polymer electrolyte fuel cells require high Pt loading. Therefore, an increase in ORR activity decreases the Pt loading required in electrocatalysts.
The combination of well-defined single-crystal electrodes modified with hydrophobic species is an effective method for enhancing the ORR1. Markovic et al. reported the structural effects on the ORR on the low-index planes of Pt in 0.1 M HClO4: Pt(100) < Pt(111) < Pt(110). Feliu et al. demonstrated that the ORR activity increases with increasing step atom density using high-index planes of Pt. A systematic study of the ORR using the high-index planes of Pt revealed that the (111) terrace edge enhances the ORR activity of Pt electrodes. Markovic et al. reported that Pt oxides are blocking species of the ORR. Infrared reflection absorption spectroscopy (IRAS) and surface-enhanced Raman spectroscopy showed that the ORR activity decreases with the increase of the band intensity of PtOH, verifying that PtOH is the main blocking species of the ORR over Pt single-crystal electrodes. Density functional theory (DFT) calculations predicted that the (111) terrace edge degrades the structure of water, decreasing the coverage of blocking species of the ORR, such as PtOH and PtO. The formation of PtOH requires water molecules around the Pt surface, as follows: Pt + H2O → PtOH + H+ + e−. Hydrophobic species expel water around the electrode surface, which may enhance the ORR activity.
Modification with alkyl amines, melamine, and protonic ionic liquids increases the ORR activity of Pt nanoparticles, as well as those of polycrystalline and single-crystal Pt electrodes. The effects of the hydrophobic species on the ORR activity significantly depend on the surface structure of the Pt electrode. The degree of activity enhancement is the highest on the Pt(111) surface of single-crystal Pt, whereas the Pt(100) surface remains unaffected, although it is sometimes deactivated by hydrophobic species. Among the hydrophobic species examined, Tetra‒n‒hexylammonium cation (THA+) has the strongest influence on Pt(111), resulting in an 8-fold enhancement in the ORR activity of Pt(111) compared with that of unmodified Pt(111).
Caffeine is less toxic than other hydrophobic species, and it activates the hydrogen evolution and oxidation reactions of Pt nanoparticles and caffeine doped carbons. Caffeine is used as a capping agent and a structure directing agent for the preparation of well dispersed Pd–Au nanochain networks with unique structures, and enhances the ORR activity. The ORR activity of caffeine derived graphene-wrapped Fe3C nanoparticles is higher than that of Pt/C in alkaline solution. However, the effects of caffeine itself on the ORR have not been examined on single crystal electrodes thus far. In this study, the structural effects of single-crystal Pt electrodes modified with caffeine on the ORR are investigated. In addition, the enhancement mechanism is investigated using IRAS.
Fuel cells, while promising, are expensive due to high catalyst requirements to increase the oxygen reduction reaction (ORR) activity. In a breakthrough, researchers found that adding caffeine to the electrodes can improve the ORR activity of platinum electrodes 11 times. This discovery can enhance the efficiency of the fuel cell, reduce the requirement for excess platinum catalysts, and ultimately lead to cheaper and more efficient fuel cells.
With global goals to transition away from fossil fuels, fuel cells stand out as a promising carbon-free energy source. Comprising an anode and a cathode separated by an electrolyte, fuel cells convert the chemical energy of fuel directly into electricity. The anode receives the fuel, while an oxidant, typically oxygen from the air, is introduced at the cathode. In a hydrogen fuel cell, hydrogen undergoes oxidation at the anode, producing hydrogen ions and electrons. The ions move through the electrolyte to the cathode, and electrons flow through an external circuit, generating electricity. At the cathode, oxygen combines with the hydrogen ions and electrons, resulting in water as the sole byproduct.
However, the presence of water affects the performance of the fuel cell. It reacts with the platinum (Pt) catalyst, forming a layer of platinum hydroxide (PtOH) on the electrode, which obstructs the efficient catalysis of the oxygen reduction reaction (ORR), leading to energy losses. To maintain efficient operation, fuel cells require a high Pt loading, which significantly increases the costs of fuel cells.
Now, in a study published in the journal Communications Chemistry on February 3, 2024, Professor Nagahiro Hoshi, along with Masashi Nakamura, Ryuta Kubo, and Rui Suzuki, all from the Graduate School of Engineering at Chiba University, Japan, have found that adding caffeine to certain platinum electrodes can increase the activity of the ORR. This discovery can potentially reduce platinum requirements, making fuel cells more affordable and efficient.
“Caffeine, one of the chemicals contained in coffee, enhances the activity of a fuel cell reaction 11-fold on a well-defined Pt electrode of which atomic arrangement has a hexagonal structure,” said Prof. Hoshi.
To assess caffeine’s impact on the ORR, researchers measured current flow through platinum electrodes immersed in an electrolyte containing caffeine. These platinum electrodes had surface atoms arranged in specific directions, namely (111), (110), and (100). There was a notable improvement in the electrode’s ORR activity with increased caffeine concentration in the electrolyte. Caffeine, when present, adsorbs onto the electrode’s surface, effectively preventing hydrogen adsorption and the formation of Pt oxide on the electrode.
However, the effect of the caffeine depended on the orientation of the platinum atoms on the electrode’s surface.
At a caffeine molar concentration of 1 × 10−6, the ORR activity on Pt (111) and Pt (110) increased by 11 and 2.5 times, respectively, with no noticeable effect on Pt (100). To understand this difference, the researchers investigated the molecular orientation of caffeine on the electrode surface using Infrared Reflection Absorption Spectroscopy. They found that caffeine gets absorbed on Pt (111) and Pt (110) surfaces with its molecular plane perpendicular to the surface. However, on Pt (100), steric hindrances cause it to be attached with its molecular plane tilted relative to the surface of the electrode.
“The increased ORR activity of Pt (111) and Pt (110) was attributed to the decreased PtOH coverage and lower steric hindrance of the adsorbed caffeine. Conversely, for Pt (100), the effect of decreasing PtOH was counteracted by the steric hindrance of the adsorbed caffeine, and thus caffeine did not affect the ORR activity,” said Prof. Hoshi.
Unlike batteries with limited lifespans, fuel cells can generate power if fuel is supplied, making them suitable for various applications, including vehicles, buildings, and space missions. The proposed method has the potential to improve the designs of fuel cells and lead to their widespread use.
References:https://doi.org/10.1038/s42004-024-01113-6
