
Healthcare in 2025 sits at the cutting edge of a tech revolution. Artificial intelligence now diagnoses illnesses in seconds, while surgeons rely on augmented reality for greater precision during complex procedures. These once-futuristic technologies now improve healthcare by helping doctors provide faster, more accurate care every day. They also let patients control their health data and manage outcomes more confidently. This article features real-world case studies showing how innovations deliver real impact. It avoids generic trend lists and focuses on lesser-known but important breakthroughs. Read on to see how these tools reshape healthcare for providers and patients in 2025 and beyond.
From wearable biosensors to robotic logistics and AI-led diagnostics, healthcare in 2025 is smarter, faster, and more resilient. PatSnap Eureka empowers R&D and healthcare teams to discover, validate, and implement innovations at record speed. Explore the Eureka platform to see how your research and development strategy can move forward with confidence.
Artificial Intelligence and Machine Learning in Healthcare
One of the most transformative trends in healthcare is the rise of artificial intelligence (AI) and machine learning. AI is now being applied across medical disciplines – from imaging and diagnosis to drug discovery and hospital operations. In 2025, advanced AI algorithms are helping doctors make faster and more accurate decisions, while also taking over routine tasks to free up clinicians for patient care.
AI for diagnosis and decision support
Machine learning models can analyze medical images like X-rays, CT scans, and MRIs with astonishing speed and accuracy. AI helped radiologists during the COVID-19 pandemic by scanning lung CT images for pneumonia linked to the virus. These systems quickly flagged patterns, easing workloads and speeding up diagnoses. Today, FDA-approved AI tools detect diabetic retinopathy from eye exams and identify signs of strokes or cancers in scans. These algorithms act as a second set of eyes, enhancing diagnostic accuracy. They excel at spotting subtle patterns that doctors might miss. Physicians still make the final call, but AI now plays a key role in early disease detection and medical imaging.
AI-powered drug discovery
Beyond diagnostics, AI is revolutionizing how new treatments are found. Traditional drug discovery takes years and costs millions, but machine learning can drastically speed up the process. AI models quickly scan massive chemical libraries to predict which molecules could work as medicines. For example, MIT researchers used AI to discover halicin, a new antibiotic effective against drug-resistant bacteria. The AI analyzed over 100 million molecules in just days, a task impossible by hand. Halicin then proved successful in lab and animal tests. This approach accelerates the development of antibiotics, antivirals, and other treatments by finding promising compounds much faster than traditional methods.
Virtual health assistants and administrative AI
Healthcare also benefits from AI in more behind-the-scenes ways. Generative AI, like GPT-4, can write clinical notes, summarize patient histories, and explain conditions in simple language. GPT-4 has even passed medical licensing exams, scoring in the top 10% of test-takers. While it lacks real-world clinical judgment, it shows strong potential as a medical assistant. Many hospitals now use “ambient listening” AI in exam rooms to record conversations and create notes automatically. By 2024, this tool became common, cutting hours of documentation time for doctors. Physicians say AI scribes reduce “pajama time” and ease burnout. At the same time, AI chatbots help patients by answering questions, checking symptoms, and offering mental health support 24/7.

Telemedicine and Remote Care
Not long ago, seeing a doctor typically meant an in-person visit. In recent years, and especially after the global pandemic, telemedicine (or telehealth) has gone mainstream. Telemedicine uses video calls, phone, and messaging to connect patients and clinicians, enabling care at a distance. In 2025, telehealth has become a standard part of healthcare delivery, improving access and convenience for many patients.
The boom of virtual visits
During the COVID-19 pandemic, virtual doctor visits skyrocketed out of necessity. While some of that spike tapered off post-pandemic, telehealth usage has stabilized at a much higher level than before. Telehealth made up just 0.1% of outpatient visits in 2019. By 2023, it had jumped to about 17%. This shift shows how fast digital care gained acceptance. Even after clinics reopened, patients found video calls useful for routine checkups and mental health sessions. Providers also saw benefits in using virtual visits for follow-ups and medication renewals. CDC data shows around 30% of U.S. adults used telemedicine in 2022. That number dropped slightly from 2021’s 37% peak. However, it still remains far higher than before 2020. This trend proves virtual care is here to stay. Put simply, virtual care is here to stay as a convenient option.
Beyond video chats – remote monitoring
Telemedicine isn’t just about talking to your doctor on Zoom. Increasingly, it’s coupled with remote patient monitoring. Patients can use at-home medical devices that automatically send data to their providers. For example, a person with hypertension might use a smart blood pressure cuff daily, or a diabetic patient could wear a continuous glucose monitor – with readings sent electronically to their clinic. Doctors or AI systems can then watch for concerning trends and intervene early. This is enabling new models like “hospital at home,” where certain patients are monitored and treated at home with hospital-level oversight via technology. In one integrated care system, primary care telehealth visits increased from 18% of visits in 2019 to 32% in 2023 as in-person visits declined, showing how care models are shifting to hybrid approaches.
Telehealth specialties and access
Telemedicine has especially flourished in certain fields. Telepsychiatry and online therapy have expanded mental health access, letting patients get counseling from the comfort of home – important for those who might hesitate to visit a clinic. Teledermatology allows dermatologists to examine skin photos or video for rashes and moles remotely. Rural areas, which may have few specialists, benefit enormously – a local clinic can facilitate a video consult with, say, an endocrinologist or cardiologist in a distant city. This “virtual specialist” model means patients don’t have to travel hours for expert care. Tele-ICU programs even enable critical care doctors to oversee patients in remote intensive care units via cameras and data feeds, ensuring smaller hospitals have 24/7 specialist input.
Wearable Health Tech and the Internet of Medical Things (IoMT)
Tiny computers on our wrists, smart patches on our skin, even connected implants – wearable health technology has exploded in popularity. These devices are part of the “Internet of Medical Things,” a network of interconnected sensors and gadgets that continuously collect health data. In 2025, wearables and IoMT devices are giving both individuals and clinicians real-time insights into health, enabling preventive care and personalized wellness like never before.
The rise of health wearables
Fitness trackers and smartwatches have graduated from counting steps to measuring serious health metrics. About 35% of U.S. adults were using wearable healthcare devices in 2023, up from 27% in 2018. These range from general devices like the Apple Watch, Fitbit, and Oura Ring to medical-grade wearables prescribed by doctors. Modern smartwatches can record an electrocardiogram (ECG), detect irregular heart rhythms (like atrial fibrillation), monitor blood oxygen saturation, track sleep stages, and even alert emergency contacts if you fall. Such features have already proven life-saving – there are multiple reports of wearables notifying people of dangerous heart arrhythmias or sudden drops in oxygen, prompting timely medical attention. Over 60% of wearable owners use their device daily , showing how quickly they’ve become part of our routines.
From consumers to patients
A key trend is the medicalization of consumer wearables. Regulatory bodies have cleared some wearable features as medical devices – for instance, the Apple Watch’s ECG and AFib detection are FDA-cleared. This blurred line means data from your everyday gadgets may end up in your official health record. Meanwhile, companies are developing patches and sensors that specifically target health needs: adhesive ECG patches that monitor cardiac patients for days or weeks (far more comfortable than a traditional Holter monitor), or wearable glucose monitors that continuously track blood sugar for diabetics without fingersticks. There are even “smart” hearing aids and earbuds that track vital signs through the ear canal. All of these devices form a network – the IoMT – sending data to cloud platforms where AI can analyze trends.
IoMT in the hospital and home
In healthcare facilities, IoMT means connected everything – beds that sense patient movement and vitals, IV pumps that report their status to a central dashboard, and wearables for inpatients that can eliminate the tangle of wires. At home, IoMT devices allow for “continuous care.” Imagine an elderly patient equipped with a set of sensors: a fall-detection pendant, a smart pill bottle that notes if doses were missed, a weight scale and blood pressure cuff that log daily readings, and motion sensors in the house to gauge activity. All these feed into an AI-driven system that can alert caregivers if something seems off (e.g. no movement in the morning could mean a fall, or gradually increasing weight could signal heart failure worsening). This kind of ambient monitoring could help seniors live independently longer while still keeping an eye on their well-being.
Data overload and privacy
With wearables and IoMT, one challenge is making sense of the flood of data. A single person’s smartwatch alone can generate thousands of data points per day. For doctors, incorporating this into care means new workflows and tools. AI comes into play here as well – for instance, algorithms can comb through a week’s worth of blood pressure readings and flag only the notable changes to the physician. Privacy is another concern: these devices collect very personal health information and often transmit it to cloud servers. Ensuring this data is secure and that users consent to how it’s used is paramount (surveys show a significant portion of consumers worry about health data privacy with apps and wearables).
Personalized Medicine and Genomics
Medicine has traditionally been one-size-fits-all – treatments developed for the “average” patient. But we now know how much individuals can differ in genetics, lifestyle, and environment. Personalized medicine (also known as precision medicine) aims to tailor healthcare specifically to you. Thanks to dramatic advances in genomics and data analytics, 2025 is seeing personalized medicine move from buzzword to bedside reality, offering treatments and prevention strategies optimized for each individual’s unique profile.
Genomic revolution
At the heart of personalized medicine is the genome – our DNA code. Two decades ago, sequencing a human genome took years and billions of dollars. Today, it’s astoundingly fast and cheap. The cost to sequence a whole genome has plummeted to around a few hundred dollars , and it keeps dropping. In fact, by 2024 Illumina (a leading sequencing company) reported a new high-throughput sequencer that can push the cost down to about $200 per genome, inching closer to the long-dreamed $100 genome . This means that genome sequencing is becoming routine – not just for rare research cases. Thousands of patients, especially those with cancer or undiagnosed diseases, are getting their genomes (or the genome of their tumor) sequenced to guide treatment.
Pharmacogenomics – the right drug for your DNA
One practical use of genomics is pharmacogenomics, which studies how genes affect drug response. We’ve learned that genetic variations can influence how you metabolize certain medications – affecting efficacy or risk of side effects. For example, about 1 in 10 people have a variant that makes the common painkiller codeine either ineffective or overly potent. In 2025, it’s increasingly common for doctors to use DNA testing to personalize prescriptions. Before prescribing an antidepressant, a physician might order a cheek swab test for genes like CYP2D6 and CYP2C19 which affect how the body processes many psychiatric meds. The test results can guide the doctor to choose a medication (or dose) that the patient’s genotype suggests will work best. Similar gene tests exist for blood thinners, cancer drugs, and more – helping avoid trial-and-error and reducing adverse reactions.
Precision oncology
Perhaps the field where personalized medicine has advanced the fastest is cancer care – often termed precision oncology. Oncologists now routinely send tumor biopsies for genomic profiling. These tests search for specific DNA mutations or biomarkers in the cancer. The results can reveal “actionable” mutations – ones that have a targeted therapy available. For instance, if a lung cancer tumor has an EGFR mutation, doctors can prescribe an EGFR inhibitor pill that specifically attacks cells with that mutation. If a breast cancer shows HER2 gene amplification, targeted antibodies like trastuzumab can be used. There are dozens of such matchups now: ALK and ROS1 gene fusions matched to certain lung cancer drugs, BRCA mutations in ovarian cancer matched to PARP inhibitor drugs, and so on. By matching the right drug to the right patient, outcomes improve; patients spared ineffective treatments and unnecessary toxicity.
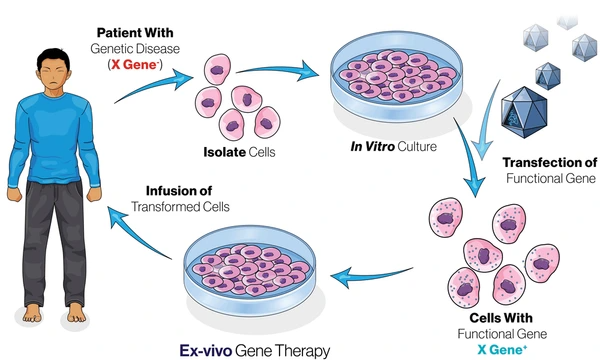
Gene therapy and editing in practice
The ultimate personalized treatment is one that fixes a problem in a patient’s own DNA. Gene therapies – where a correct copy of a gene is delivered to a patient’s cells – have matured after decades of research. Recently, several gene therapies have been approved for hereditary diseases. Notably, in late 2023 the FDA approved the first CRISPR-based gene therapy for sickle cell disease. This therapy (Casgevy™) uses the CRISPR-Cas9 gene-editing system to modify a patient’s bone marrow stem cells, correcting the genetic defect that causes sickle cell. It was a milestone: the first treatment that literally edits a patient’s genome to cure a disease. Another gene therapy (lovo-cel, trade name Lyfgenia) was approved around the same time for sickle cell using a lentiviral vector. For patients, this means a one-time treatment could potentially eliminate a disease they’ve battled their whole lives. Beyond sickle cell, gene therapies exist for inherited blindness, spinal muscular atrophy in infants, and more are in development. CRISPR gene editing is being tested for conditions from beta thalassemia to certain cancers, opening a new era of molecular cures.
Holistic personalization – beyond genetics
While genomics is a big piece of personalized medicine, it’s not the only piece. True personalization combines genetics with other data – environment, lifestyle, microbiome, etc. Two people may share a genetic risk for diabetes, but lifestyle differences can lead to very different outcomes. One person might eat well and stay active. The other could have a poor diet and little exercise. As a result, their health risks and disease progression can vary greatly. Modern health systems now use lifestyle and social data in care plans. This helps tailor diet and exercise advice to real-life behaviors. Patients in less healthy environments may get extra support and guidance. Even our gut bacteria, or microbiome, vary from person to person. These differences can impact immunity and drug response. Soon, treatments may adjust based on a person’s microbiome profile.
Biotechnology Breakthroughs and Novel Therapies
Hand-in-hand with personalized medicine, the biotechnology field is delivering remarkable new therapies that were unimaginable not long ago. From editing genes to reprogramming cells, these biotech breakthroughs are addressing diseases at their root cause. In 2025, we’re witnessing the coming-of-age of technologies like gene editing, cell therapy, and mRNA platforms, which together promise cures for once incurable conditions.
CRISPR and gene editing therapies
As mentioned, CRISPR-Cas9 gene editing has made the leap from lab bench to clinic. The approval of a CRISPR-based sickle cell therapy is a pivotal moment. Beyond that, CRISPR is being explored for other blood disorders (like beta thalassemia), genetic forms of blindness, and even to make immune cells better at fighting cancer. Scientists have launched trials using CRISPR to edit immune T-cells to more efficiently target tumors. Another line of research uses gene editing tools to disrupt genes in the liver that cause high cholesterol – potentially offering a “one-and-done” treatment to permanently lower a person’s heart disease risk. While CRISPR grabs headlines, other gene editing techniques (TALENs, base editors, prime editors) are also advancing, some with more precision to minimize off-target effects. We are still in early days, but the ability to rewrite DNA in living patients could eventually cure thousands of genetic diseases.
mRNA vaccines and therapeutics
The COVID-19 pandemic in 2020 catapulted mRNA technology into the spotlight with the success of Pfizer-BioNTech and Moderna’s vaccines. Those vaccines proved that messenger RNA, delivered via lipid nanoparticles, can be a safe and effective platform . Now in 2025, mRNA research is booming. Scientists are developing mRNA vaccines for other infectious diseases (from influenza to HIV) and even for cancer.
For instance, trials are underway for personalized mRNA cancer vaccines – a patient’s tumor is sequenced, and an mRNA vaccine is custom-made to encode tumor-specific mutant proteins, training the immune system to attack the cancer. Early results in melanoma patients have been promising. Beyond vaccines, mRNA is being used to instruct cells to produce therapeutic proteins. An exciting area is using mRNA to spur regeneration: one study injected mRNA encoding a regenerative factor into heart attack patients’ hearts to promote tissue repair.
Since mRNA can be made rapidly once you know the sequence, it makes drug development much more agile. The pandemic showed that – a new vaccine was designed in days once the virus genome was known. With improved lipid nanoparticle delivery systems (the tiny fat bubbles that protect mRNA in the body), which have been refined over decades , we can now target mRNA to specific organs. The success of mRNA vaccines truly validated nanomedicine approaches: “Fragile mRNA molecules can’t get into cells on their own; they owe their success to lipid nanoparticles” that deliver them . This success is inspiring a generation of mRNA therapies on the horizon.
Cell therapies – CAR-T and beyond
Another biotech breakthrough area is cell therapy – treating patients with living cells. The poster child here is CAR-T therapy for cancer. CAR-T involves taking a patient’s own T-cells (a type of immune cell), genetically modifying them in the lab to better recognize cancer (by giving them a synthetic receptor, the Chimeric Antigen Receptor), and infusing them back. CAR-T therapies have had stunning success in certain leukemias and lymphomas, even curing patients who had no other options. By 2025, several CAR-T products are on the market for blood cancers and being researched for solid tumors. Scientists are also working on “off-the-shelf” CAR-T, using donor cells or gene-edited cells that could be used in any patient, which would simplify the process. Beyond CAR-T, there are other cell therapies: for example, modified stem cells for regenerative medicine.
RNA therapies and gene silencing
Apart from CRISPR and mRNA, another class of biotech therapies is RNA interference (RNAi) and antisense oligonucleotides – essentially “gene-silencing” drugs. These are lab-made RNA snippets that can shut down the expression of a harmful gene. A few of these are already FDA-approved (for example, a drug that silences a gene to treat hereditary high cholesterol). They open yet another avenue to target diseases at a genetic/molecular level without altering DNA. As delivery improves (often using lipid nanoparticles like mRNA), more such therapies for conditions like Huntington’s disease or ALS are in development.
Organoids and lab-grown organs
On the futuristic end, biotech researchers are growing tiny versions of human organs in the lab from stem cells – called organoids. We have mini brains, livers, kidneys, and more being grown for research. While using them for transplants is far off, organoids are incredibly useful for personalized medicine testing (e.g. testing multiple drugs on a patient’s tumor organoid to see which works best) and for studying diseases in human-like models. In parallel, tissue engineering advances aim to 3D-print organs or tissue patches. Scientists have bioprinted rudimentary organs like mini-hearts and are working toward printing functional kidney or liver tissue. The dream is that one day, organ transplant waiting lists could be eliminated by growing replacement organs from a patient’s own cells.
Case Insight: Dr. Emily Carter, a senior research scientist, cut her development time in half using Eureka’s cross-industry insights in nanoparticle dispersion from cosmetics to apply in mRNA vaccine delivery.
Robotics in Surgery and Healthcare
Robots have firmly planted themselves in the operating room and hospital corridors. Medical robotics is a fast-evolving field, ranging from surgical robots that enable ultra-precise operations to service robots that deliver supplies. In 2025, robots are extending the capabilities of human caregivers, enhancing precision, and taking over repetitive tasks – all aimed at improving patient outcomes and operational efficiency.
Surgical robots – a steady hand
The da Vinci Surgical System is the most recognized robotic platform in modern surgery. Surgeons use it to perform minimally invasive procedures with enhanced precision. The surgeon sits at a console and controls robotic arms holding tiny instruments inside the patient. The system translates each hand movement into smaller, steadier motions. This control allows for more accurate dissection than a human hand might achieve alone. Since its debut over 20 years ago, da Vinci has become widely adopted worldwide. By the mid-2020s, doctors had used it in more than 12 million procedures. These include surgeries in urology, gynecology, cardiac care, and general surgery. Patients benefit from smaller incisions, reduced pain, and faster recovery. By 2025, many hospitals operate multiple robotic systems. For certain procedures like prostate removal, surgeons now rely almost entirely on robotic assistance.
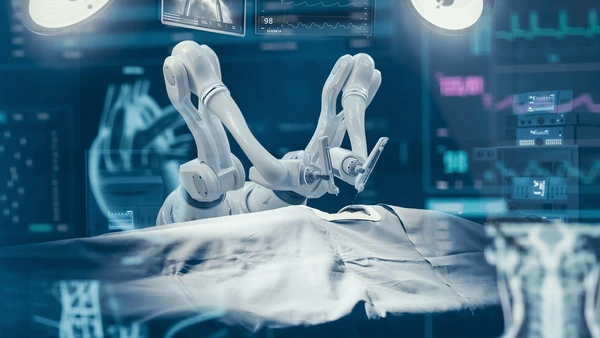
Beyond the cutting – robotic support roles
Robotics in healthcare isn’t just about cutting or suturing. There is a growing fleet of clinical service robots designed to handle routine chores in medical facilities. For example, hospitals are employing autonomous robotic carts or humanoid robots to ferry medications, lab samples, and linens around the hallways. These robots use guided mapping and even computer vision to navigate from the pharmacy to a nursing station, or from the lab to the ward, carrying payloads that staff would otherwise have to transport. This helps mitigate workforce shortages and allows nurses and support staff to focus more on patient-facing duties instead of errands. In some facilities, cute-eyed robots named TUG or Moxi roam the halls performing delivery tasks and have become friendly sights for staff. Likewise, in pharmacies, robotic dispensing systems automatically sort and prepare prescriptions with high accuracy.
Driving Healthcare Innovation Forward with Patsnap Eureka AI Agents
Try Patsnap Eureka AI Agent, Accomplish Your Tasks Faster
As emerging healthcare technologies reshape patient care in 2025, the pace of discovery, regulatory demands, and cross-disciplinary knowledge are accelerating like never before. From AI-powered diagnostics and telemedicine to biosensors and precision therapeutics, staying competitive requires more than intuition—it requires smart tools. That’s where Patsnap Eureka and its suite of AI agents come into play.
Designed to automate and accelerate every stage of healthcare R&D, Eureka AI agents help teams search smarter, analyze faster, and innovate more confidently.

Accelerate Biomedical Research and Diagnostic Breakthroughs
For healthcare startups and research institutions developing AI-driven imaging diagnostics or wearable health monitors, the Find Solution agent quickly analyzes complex technical problems and presents proven solutions from global patents, literature, and best practices. Whether you’re enhancing biosensor sensitivity or refining adhesive biocompatibility, Eureka surfaces validated methods like surface modification via plasma treatment or nano-enhanced coatings, saving critical development time.
Inspire New Ideas Across Medtech Development
Healthcare R&D is often fueled by cross-sector innovation. Patsnap Eureka’s AI agents excel in uncovering connections across industries—perfect for inspiring new directions in digital therapeutics, implantable devices, or smart drug delivery systems. Using the platform, users like Robert, a product design specialist, discovered ideas that exceeded expectations and pushed the limits of their team’s product development.
Streamline Risk Analysis in Medical Device Design
Safety is paramount in medical innovation. Whether you’re engineering a next-gen insulin pump, cardiac monitor, or neurostimulation device, Eureka’s D-FMEA and D-FMEA Analytics agents automate design failure mode and effects analysis—flagging potential issues and improving compliance documentation. Instead of weeks of manual checking, Eureka delivers precise, high-quality reports in minutes, trusted by R&D managers in the medical field.
Unlock Faster Material Discovery for Biocompatible Solutions
Material selection is often the bottleneck in healthcare product innovation—especially for skin-contact wearables, surgical adhesives, or drug-eluting implants. With Material Composition Analysis, Patsnap Eureka scans databases with just a few inputs, instantly delivering material candidates matched to the required properties such as hydrogel flexibility, thermal resistance, or tissue compatibility.
Dr. Emily Carter, a senior research scientist, highlighted the time-saving power of this tool, calling it a true game-changer for driving innovation in healthcare materials research.
Learn Cross-Industry Insights for Smarter Healthcare Strategies
Innovation in healthcare isn’t just about the lab—it’s about learning from industries like aerospace, consumer electronics, and advanced manufacturing. Eureka provides a knowledge-driven approach to healthcare R&D, equipping professionals with curated technical know-how, case studies, and solution maps tailored to healthcare’s most pressing challenges.
As Amanda, an R&D manager for unmanned aerial vehicles, noted, Eureka helps users move from trial-and-error to a structured, fact-based approach—a mindset shift that is just as valuable in healthcare innovation.
Conclusion
The landscape of healthcare technology in 2025 is dynamic and exhilarating. We’re at a point where science fiction is steadily transforming into clinical fact. Doctors are diagnosing diseases with AI’s help, operating with robotic precision, treating patients with genetic and cellular therapies, and leveraging data from wearables and digital twins to make informed decisions. Patients, in turn, are benefiting from more accessible care through telemedicine, greater personalization of treatments, and new hope for conditions once deemed incurable.
In conclusion, the emerging technologies of 2025 are revolutionizing healthcare on multiple fronts. They are enabling us to predict illnesses earlier, personalize treatments better, perform procedures safer, and empower patients further. It’s an exciting time to be in the intersection of health and technology. The ultimate promise is healthier lives and longer lifespans with a better quality of life – a world where technology and compassion together drive healing. While challenges remain, the innovations described in this article provide plenty of reason for optimism. The future of healthcare is being written today in code, silicon, and biology – and it’s a story of hope and progress for all of us.
To get detailed scientific explanations of Top Emerging Healthcare Technologies, try Patsnap Eureka.


