
A pi bond (π bond) is a fundamental type of covalent bond in chemistry that arises from the side-by-side overlap of atomic orbitals. It plays a key role in the structure and reactivity of molecules containing double or triple bonds and is essential to understanding organic compounds, resonance, and molecular geometry.
What are pi bonds? Eureka Technical Q&A explains that pi bond form from the sideways overlap of p orbitals—typically alongside a sigma bond—contributing to double and triple bonds in molecules like ethene and acetylene, and affecting molecular reactivity and shape.
In this article, we’ll explore what a pi bond is, how it forms, and how it differs from a sigma bond, supported by real-world examples in organic and inorganic chemistry.
What Is a Pi Bond?
A pi bond is a covalent bond formed when two adjacent p orbitals overlap laterally (side-to-side) above and below the internuclear axis. This type of bonding does not occur along the line connecting the two nuclei — instead, electron density is concentrated in lobes above and below the bond axis.
Pi bonds are characterized by the overlap of parallel p orbitals, which results in a bond that is less localized than sigma bonds. This overlap creates a nodal plane that includes the line of the bond axis, with the shared electrons occupying regions above and below this plane . The presence of pi bonds contributes to the overall shape and reactivity of molecules, often leading to delocalized electron systems in conjugated compounds.
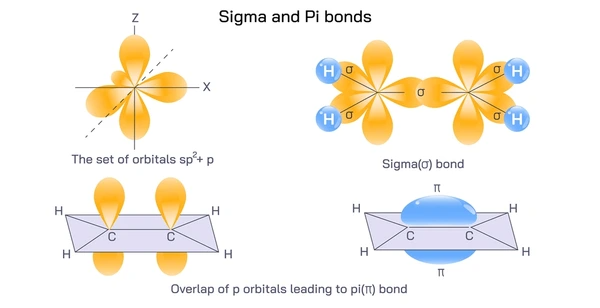
Structure of Pi Bonds
- Involves unhybridized p orbitals (usually 2p).
- Each pi bond is made of two lobes of electron density.
- The bond lies above and below the plane of the atoms.
- It restricts rotation around the bond axis.
This unique geometry results in weaker bonding than sigma bonds, since the overlap is less direct.
How Pi Bonds Form
Step-by-Step Formation:
- Sigma Bond Formation:
- First, atoms form a sigma (σ) bond via end-to-end overlap (usually s-s, s-p, or p-p).
- This establishes the initial bond framework.
- Pi Bond Formation:
- Remaining unhybridized p orbitals on each atom overlap side-by-side.
- This forms the pi bond above and below the sigma bond.
Key Points:
- Pi bonds cannot exist alone — a sigma bond must be present.
- Only orbitals that are parallel and unhybridized can form pi bonds.
- Multiple bonds arise from pi bonding.
Pi Bonds vs. Sigma Bonds
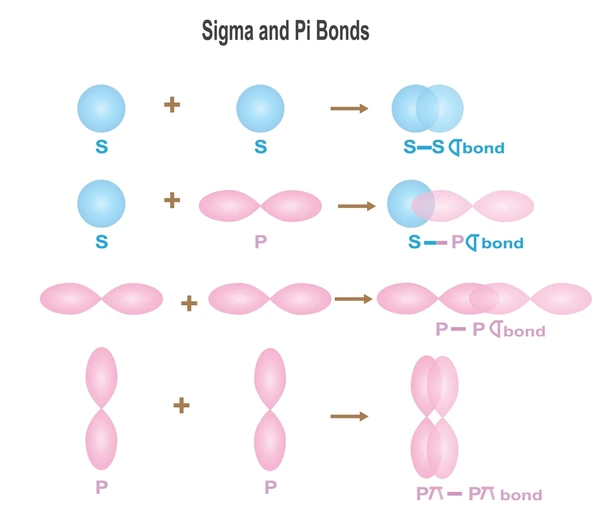
Definition and Formation
- Sigma (σ) Bonds: These are formed by the end-to-end overlap of atomic orbitals. This type of bond allows for cylindrical symmetry around the bond axis, making sigma bonds strong and capable of holding two atoms together in a single covalent bond.
- Pi (π) Bonds: These are formed by the side-by-side overlap of parallel p orbitals. Pi bonds have a nodal plane that includes the bond axis, which contributes to their distinct electronic structure compared to sigma bonds.
Strength and Characteristics
- Bond Strength: Sigma bonds are generally stronger than pi bonds due to their direct overlap of orbitals. This strength contributes to the stability of molecules where sigma bonds are present.
- Bond Length: Sigma bonds typically result in shorter bond lengths compared to pi bonds. This is because the orbital overlap in sigma bonds is more direct and efficient.
- Reactivity: Pi bonds are often more reactive than sigma bonds, especially in unsaturated systems like alkenes and alkynes. This reactivity is due to the presence of a nodal plane in pi bonds, which makes them more susceptible to reactions.
Role in Molecules
- Conjugated Systems: In conjugated systems, pi bonds can delocalize electrons over multiple bonds, leading to increased stability and unique properties, such as those seen in aromatic compounds like benzene.
- Bond Alternation: In conjugated hydrocarbons, sigma and pi bonding can exhibit bond alternation or equalization, depending on the system. Sigma bonds can partly compensate for the alternation in pi bonding, influencing the overall molecular structure.
Applications and Examples
- Aromatic Compounds: The delocalization of electrons in conjugated pi bonds is crucial for the stability and reactivity of aromatic compounds. This concept is known as resonance stabilization.
- Radical Chemistry: In radical chemistry, sigma and pi bonds play a role in the formation and reactivity of radicals. For instance, the sigma-pi coupling in diradicals like benzynes can affect their aromaticity and reactivity.
| Property | Pi Bond (π) | Sigma Bond (σ) |
|---|---|---|
| Orbital Overlap | Side-by-side (lateral) | Head-on (end-to-end) |
| Orbital Types | Unhybridized p orbitals | s, p, or hybrid orbitals |
| Location of Density | Above and below internuclear axis | Along internuclear axis |
| Strength | Weaker | Stronger |
| Can Exist Alone? | No | Yes |
| Rotational Freedom | Restricted | Free rotation |
Examples of Pi Bonds

1. Ethene (C₂H₄)
- Contains a C=C double bond.
- One sigma bond + one pi bond between the two carbon atoms.
- The pi bond restricts rotation, making the molecule planar.
2. Ethyne (C₂H₂)
- Contains a C≡C triple bond.
- One sigma bond + two pi bonds.
- The two pi bonds are mutually perpendicular, giving a linear structure.
3. Oxygen Molecule (O₂)
- Double bond: 1 sigma + 1 pi bond.
- The pi bond accounts for the magnetic behavior of O₂ due to unpaired electrons in pi* orbitals.
4. Nitrate Ion (NO₃⁻)
- Resonance structures contain delocalized pi bonding.
- Electrons are shared across the oxygen atoms through overlapping p orbitals.
Role of Pi Bonds in Molecular Geometry
- Restrict rotation, leading to cis-trans isomerism in alkenes.
- Influence bond length: double bonds are shorter than single bonds.
- Affect reactivity: pi bonds are electron-rich and susceptible to electrophilic attack.
Importance of Pi Bonds in Chemistry
- Organic synthesis: Pi bonds are sites of reactivity in many chemical reactions (e.g., addition, substitution).
- Spectroscopy: Pi-electron systems absorb UV/Vis radiation, used in detecting compounds.
- Conjugation: Alternating pi bonds increase stability and lower HOMO–LUMO gaps, as seen in benzene.
Pi Bonds and Resonance
In systems with alternating double and single bonds (e.g., benzene, carboxylates), pi bonds delocalize, creating resonance structures and enhanced stability.
Example:
- Benzene (C₆H₆) has six pi electrons delocalized over six carbon atoms.
- The pi system contributes to its aromaticity and chemical inertness.
Conclusion
Pi bonds are essential components of multiple bonding in chemistry, formed through side-by-side orbital overlap. While weaker than sigma bonds, they play a key role in defining molecular structure, stability, and reactivity — especially in unsaturated hydrocarbons and resonance-stabilized molecules.
From alkenes and alkynes to aromatic systems, understanding pi bonds unlocks deeper insights into molecular behavior and reactivity patterns in both organic and inorganic chemistry.
FAQs
Typically, unhybridized 2p orbitals overlapping side-by-side.
Yes. Double bonds have 1 pi bond, and triple bonds have 2 pi bonds.
Because the side-by-side overlap is less effective than head-on overlap, leading to less orbital overlap.
No. A sigma bond must exist for a pi bond to form.
They restrict rotation due to the fixed alignment of p orbitals.
To get detailed scientific explanations of Pi Bonds, try Patsnap Eureka.


