
Amine compounds are a vital group of organic molecules that feature nitrogen atoms. They play critical roles in both biological functions and industrial applications. You can find amines in amino acids, neurotransmitters, dyes, pharmaceuticals, and polymers. Due to their unique structure and reactivity, every amine serves as a building block in organic chemistry and modern manufacturing processes.
What is an amine in chemistry? Eureka Technical Q&A explains that amines are nitrogen-containing organic compounds, classified as primary, secondary, or tertiary based on their structure—playing key roles in pharmaceuticals, dyes, and biological systems due to their basicity and reactivity.
In this article, we’ll explore the structure of amines, their classification, key chemical and physical properties, and their importance in organic and applied chemistry.
What Are Amines?
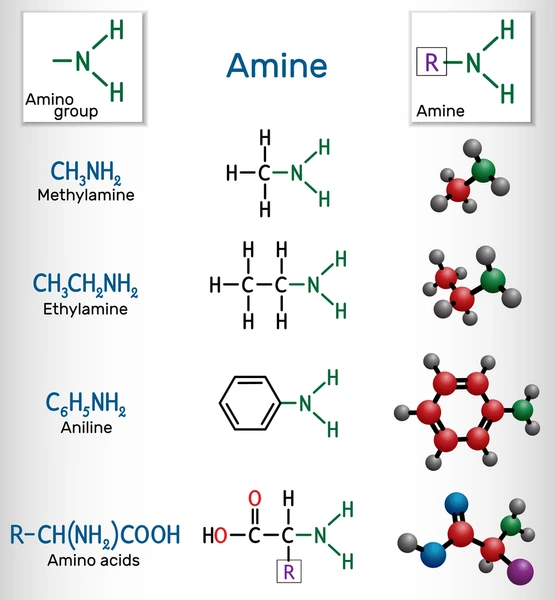
Amines are derivatives of ammonia (NH₃) in which one or more hydrogen atoms are replaced by alkyl or aryl groups. Like ammonia, amines have a lone pair of electrons on the nitrogen atom, which gives them basic properties and the ability to act as nucleophiles.
General formula: RₙNH₍₃₋ₙ₎
Where R = alkyl or aryl group, and n = 1, 2, or 3.
Classification of Amines
Amines are classified based on the number of organic groups attached to the nitrogen atom.
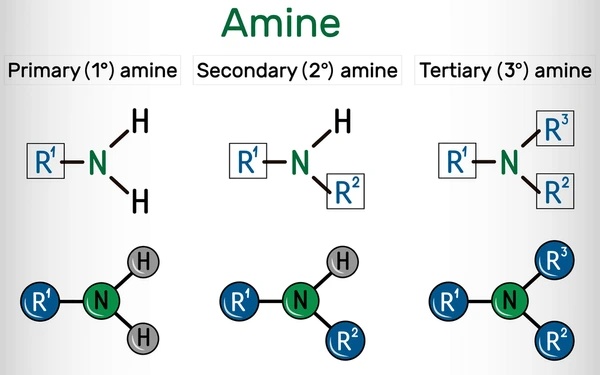
1. Primary Amines (1°)
- One alkyl or aryl group attached to the nitrogen.
- General formula: R–NH₂
- Example: Methylamine (CH₃NH₂)
2. Secondary Amines (2°)
- Two alkyl or aryl groups attached.
- General formula: R₂NH
- Example: Dimethylamine ((CH₃)₂NH)
3. Tertiary Amines (3°)
- Three organic groups bonded to nitrogen.
- General formula: R₃N
- Example: Trimethylamine ((CH₃)₃N)
4. Quaternary Ammonium Compounds
- Four organic groups attached to nitrogen, carrying a positive charge.
- General formula: R₄N⁺
- Example: Tetramethylammonium chloride ((CH₃)₄N⁺Cl⁻)
Structure and Hybridization of Amines
- The nitrogen atom in amines is sp³ hybridized, forming a trigonal pyramidal geometry.
- The lone pair on nitrogen significantly affects molecular polarity and reactivity.
- The bond angles in amines are typically slightly less than 109.5° due to lone pair repulsion.
Physical Properties of Amines
| Property | Description |
|---|---|
| Boiling Point | Amines have higher boiling points than alkanes due to hydrogen bonding (in 1° and 2° amines) but lower than alcohols. |
| Solubility | Lower amines are soluble in water due to hydrogen bonding; solubility decreases with increasing chain length. |
| Odor | Many amines have strong, fishy odors, especially volatile aliphatic amines. |
| Polarity | Amines are polar compounds, enhancing their solubility in polar solvents. |
Basicity of Amines
Amines are basic because of the lone electron pair on nitrogen, which can accept protons (H⁺).
Factors Influencing Basicity:
- Electron-donating groups (EDGs) increase basicity.
- Aromaticity in aryl amines decreases basicity due to delocalization.
- Solvation effects in water can stabilize conjugate acids and affect basic strength.
Example:
- Aliphatic amines > Ammonia > Aniline (C₆H₅NH₂) in basicity
Chemical Reactions of Amines
1. Alkylation
Amines react with alkyl halides to form higher amines or quaternary ammonium salts.
2. Acylation
Primary and secondary amines react with acid chlorides or anhydrides to form amides.
3. Reaction with Nitrous Acid
- Primary aliphatic amines: form alcohols (via diazonium salts).
- Primary aromatic amines: form stable diazonium salts used in dye synthesis.
- Secondary amines: form N-nitrosoamines.
- Tertiary amines: undergo salt formation or no reaction.
4. Hofmann Elimination
Quaternary ammonium salts can undergo Hofmann elimination to produce alkenes.
Aromatic Amines vs Aliphatic Amines
Chemical Structure
- Aromatic amines: Contain an amino group (-NH₂) attached to an aromatic ring. Example: Aniline.
- Aliphatic amines: Have amino groups attached to alkyl chains. Example: Methylamine.
Properties
- Aromatic amines: Less basic; higher boiling/melting points due to resonance.
- Aliphatic amines: More basic; can be volatile with strong odors.
Applications
- Aromatic amines: Used in dyes, pigments, and pharmaceutical intermediates.
- Aliphatic amines: Found in surfactants, resins, and solvents.
Safety
- Aromatic amines: Some are toxic or carcinogenic; may cause sensitization.
- Aliphatic amines: Can irritate skin and lungs; may be harmful in large doses.
| Feature | Aliphatic Amines | Aromatic Amines |
|---|---|---|
| Basicity | Stronger bases | Weaker bases (due to delocalization) |
| Reactivity | More nucleophilic | Less nucleophilic |
| Examples | Ethylamine, Propylamine | Aniline, Toluidine |
Important Amines in Biology and Industry
Biological Amines:
- Amino acids: building blocks of proteins
- Neurotransmitters: dopamine, serotonin, histamine
- Alkaloids: plant-based amines like morphine, caffeine
Industrial Uses:
- Dyes: aniline dyes
- Drugs: antihistamines, antidepressants
- Agricultural chemicals: herbicides, insecticides
- Polymers: nylon, polyurethane production
- Corrosion inhibitors and surfactants
Health and Safety Considerations
- Some volatile amines can be toxic or irritant.
- Aromatic amines like aniline are potentially carcinogenic.
- Proper ventilation and protective equipment are required when handling amines in the lab or industry.
Conclusion
Amines are versatile organic compounds with significant structural, chemical, and biological importance. Their classification into primary, secondary, tertiary, and quaternary forms allows for diverse chemical behavior. Whether in the lab, nature, or industrial settings, understanding the structure, reactivity, and properties of amines is essential for exploring organic chemistry and designing new materials and medicines.
FAQs
The lone pair of electrons on the nitrogen atom can accept a proton, making amines act as Brønsted-Lowry bases.
Amines are derivatives of ammonia (NH₃) in which one or more hydrogen atoms are replaced with organic groups.
Lower molecular weight amines are soluble due to hydrogen bonding. Larger amines have reduced solubility.
They are commonly used as antiseptics, disinfectants, and fabric softeners.
No, aromatic amines are usually less basic and less nucleophilic due to resonance stabilization.
To get detailed scientific explanations of Amine, try Patsnap Eureka.


