
The common ion effect plays a key role in understanding how ionic compounds behave in solution. It influences solubility, pH, and chemical equilibria, making it essential for anyone studying chemistry, from students to laboratory scientists. This principle helps explain how the addition of certain ions can shift reactions, reduce solubility, and affect acid-base behavior. In this article, you’ll learn what the common ion effect is, how it works, and where it’s applied in real-world chemistry.
Definition of the Common Ion Effect
What is the common ion effect? Eureka Technical Q&A explains that it’s the shift in equilibrium that occurs when an ion common to a dissolved substance is added to the solution, reducing solubility and altering reaction behavior in chemical systems.
The common ion effect refers to the shift in equilibrium that occurs when a solution already contains an ion that is also part of a dissolved compound. When this “common ion” is added, it suppresses the ionization or solubility of a weak electrolyte or sparingly soluble salt by Le Chatelier’s Principle.
In simple terms, adding more of an ion that’s already present pushes the reaction to the left, reducing the amount of dissolved ions in the solution.
Common Ion Effect in Acid-Base Equilibria
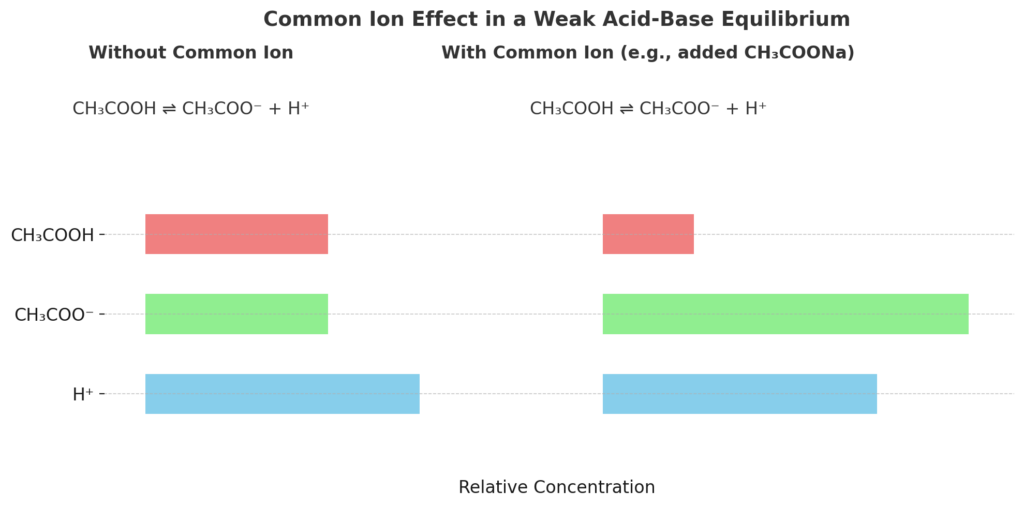
In solutions containing weak acids or weak bases, the common ion effect can significantly reduce ionization.
Example:
Consider acetic acid (CH₃COOH), a weak acid in water:
CH₃COOH ⇌ CH₃COO⁻ + H⁺
If sodium acetate (CH₃COONa), which contains the common ion CH₃COO⁻, is added to the solution, it increases the acetate ion concentration. This causes the equilibrium to shift left, reducing H⁺ concentration and raising the pH.
This is a common principle in buffer solutions, which resist changes in pH when acids or bases are added.
Common Ion Effect in Solubility
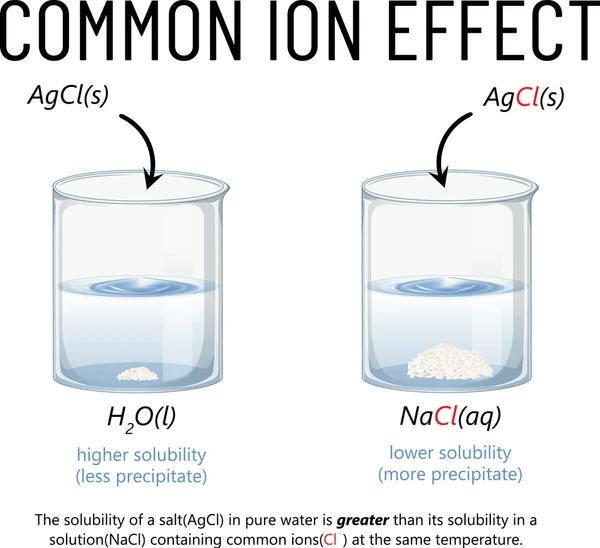
The common ion effect also affects the solubility of sparingly soluble salts.
Example:
Consider the solubility of barium sulfate (BaSO₄) in water:
BaSO₄ ⇌ Ba²⁺ + SO₄²⁻
If you add more Ba²⁺ ions, such as from BaCl₂, the reaction shifts to the left, and less BaSO₄ dissolves. This shift decreases solubility due to the common ion effect.
Mathematical Representation
The effect can be described using the solubility product constant (Ksp) or the acid dissociation constant (Ka).
For a salt AB:
AB ⇌ A⁺ + B⁻
Ksp = [A⁺][B⁻]
When more of ion A⁺ is added from another source, the equilibrium shifts left, decreasing the concentration of B⁻ and lowering the solubility of AB.
Applications of the Common Ion Effect
Chemical Industry
Chemical engineers use the common ion effect to adjust compound solubility during manufacturing. For example, they apply it in baking soda production and water treatment systems. By adding specific ions, they control salt precipitation and improve efficiency in purification processes.
Pharmaceutical Industry
Drug formulators use this effect to enhance drug solubility and bioavailability. They add salts containing ions already present in the drug compound. This shifts the solubility equilibrium, allowing more of the drug to dissolve in water-based solutions.
Electrochemistry
Battery designers use the common ion effect to stabilize electrolyte performance in sodium-ion batteries. By adding compatible ions, they maintain structural integrity and improve the battery’s cycling stability. This helps extend battery life and reliability.
Material Science
Researchers use common ion effects to control crystal shape and size during material synthesis. For instance, in calcium sulfate production, adjusting ion concentrations helps tailor crystal properties for construction, medical, or industrial applications.
Environmental Engineering
Water treatment facilities use this principle to remove contaminants effectively. By altering the ion concentration, they trigger the precipitation of pollutants, making them easier to filter and separate from clean water.
Industrial Separation Processes
Engineers use common ion effects in liquid membrane systems to separate dissolved substances. By maintaining targeted ion levels, they reduce solubility and enhance the efficiency of material recovery from complex solutions.
Chemical Synthesis
Organic chemists apply this effect to influence reaction routes and product yields. In some processes, they use it to improve metal salt solubility in ionic liquids, which supports advanced electrochemical reactions and material innovation.
Nanotechnology
In nanomedicine, scientists use common ion effects to manage nanoparticle stability. They regulate ion presence to control particle agglomeration and degradation. This ensures safer and more effective drug delivery and imaging applications.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Aqueous Na-ion Battery Ningbo University | Enhanced cycling stability with no capacity decay over 2000 cycles and outstanding rate performance due to common ion effect | Large-scale energy storage systems requiring long-term stability and high performance |
| Liquid Membrane Separation Process Esso Research & Engineering Co. | Improved purification efficiency and minimized contamination by suppressing solubility beyond traditional limits | Industrial wastewater treatment and removal of specific ions like fluoride |
| Artificial Stomach/Duodenum (ASD) Model Eli Lilly & Co. | Accurately simulates gastric environments with varying chloride levels to predict drug dissolution and absorption | Pharmaceutical research for optimizing drug formulations and predicting in vivo behavior |
| Na-Ion Desalination (NID) Cell The Board of Trustees of the University of Illinois | Achieves high desalination efficiency and water recovery with reduced energy consumption | Large-scale water desalination for drinking water production and industrial processes |
| Electrochemical Chlorine Dioxide Generator Pureline Treatment Systems LLC | Improved efficiency and lifespan by reducing hardness-causing ion concentrations in electrolyte solutions | Water treatment systems for disinfection and purification in various industries |
FAQs
What is a common ion?
A common ion is an ion that appears in more than one compound or source in a solution.
Does the common ion effect always reduce solubility?
Yes, for sparingly soluble salts, adding a common ion reduces solubility due to equilibrium shifts.
How does the common ion effect affect pH?
In weak acid or base systems, adding a common ion decreases ionization, which can shift the pH up or down depending on the system.
Is the common ion effect related to Le Chatelier’s Principle?
Yes. The principle explains how equilibrium shifts to oppose changes, such as the addition of a common ion.
Can the effect be used to purify compounds?
Yes. By reducing solubility, it can force certain salts to precipitate, allowing for selective separation.
Conclusion
The common ion effect is a fundamental chemical principle with wide-ranging applications in laboratory work, medicine, industry, and environmental science. By understanding how ions in solution influence equilibrium, chemists can predict and control reactions more effectively. Whether managing pH, solubility, or ion concentrations, the effect is a powerful tool for precision in chemistry.
To get detailed scientific explanations of common ion effects, try Patsnap Eureka.


