With ongoing breakthroughs in biotechnology, antibody-based therapeutics have emerged as one of the most promising and clinically valuable classes of molecules in modern biopharmaceuticals. From early monoclonal antibodies to today's bispecific antibodies, nanobodies, antibody-cytokine fusion proteins, and AI-designed non-natural antibodies, this drug class has undergone multiple iterations in structure, functionality, and application scope. These innovations have significantly enhanced targeting specificity, binding affinity, and therapeutic efficacy, unlocking new potential in cancer immunotherapy, autoimmune disease intervention, and infectious disease prevention.
However, as structural complexity increases, traditional R&D models face mounting challenges—low efficiency, long development cycles, high costs, and uncontrollable risks. Accelerating the design and development of next-generation antibodies while ensuring safety and druggability has become a central question in the biopharmaceutical industry. Artificial intelligence is now transforming antibody development at unprecedented speed. Through deep learning, structural prediction, functional simulation, and ADMET profiling, AI is reshaping the entire workflow. Among these tools, Eureka LS, with its powerful multimodal data integration system, demonstrates exceptional potential across the full R&D lifecycle of various antibody formats—including monoclonal antibodies (mAbs), bispecific antibodies, VHH/Nanobodies, and fusion proteins—providing a solid technological foundation for the intelligent and efficient evolution of antibody therapeutics.
Eureka LS, leveraging regulatory-approved drug and patent data from Synapse, offers companies in-depth analysis reports on antibody drug approvals and intellectual property (IP) landscapes. These reports cover basic drug information, clinical performance, intended uses, patent status, and market trends, helping users assess commercial opportunities, mitigate IP risks, and make data-driven decisions for product strategy. By intelligently analyzing efficacy, IP distribution, and competitive dynamics of globally approved antibodies, Eureka LS enables informed choices in project initiation, licensing, tech transfer, and biosimilar development—thereby boosting success rates and ROI.
Moreover, Eureka LS expedites candidate antibody screening and prediction processes. By analyzing target popularity, indication expansion trends, and technological evolution, the platform helps identify emerging R&D hotspots and market gaps, offering early guidance for strategic innovation. This comprehensive, data-driven solution reduces costs and failure risks while ensuring smooth project progression and maximizing commercial value.
Definition of Antibody Drugs
Antibody drugs are therapeutic immunoglobulin molecules generated via genetic engineering or hybridoma techniques. These molecules can specifically recognize and bind to target antigens (e.g., tumor-associated antigens, inflammatory cytokines, or pathogen surface proteins), exerting effects such as neutralization, signal blockade, induction of cell death, or immune system activation. Widely used in oncology, autoimmune diseases, infectious diseases, and cardiovascular conditions, antibody drugs represent one of the fastest-growing and most promising therapeutic categories in biopharma today.
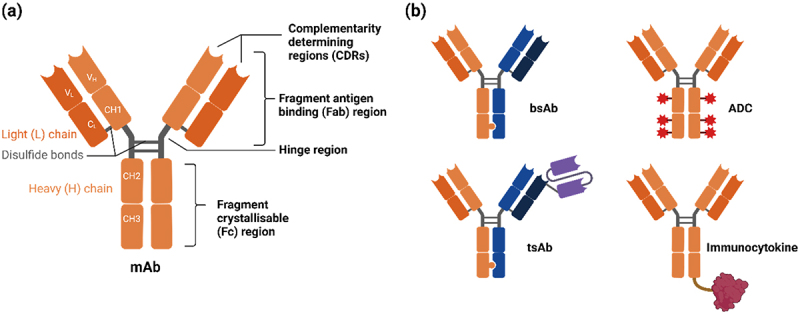
Typically, antibody drugs adopt the IgG immunoglobulin structure, comprising two identical heavy chains and two identical light chains, stabilized by inter-chain disulfide bonds. Each chain includes a variable region (responsible for antigen recognition) and a constant region (conferring structural stability and immune functionality). This structural framework allows for further modifications, such as humanization, fragmentation, or bispecific engineering.
Functionally, antibody activity is primarily governed by two regions:
·Fab region (Fragment antigen-binding): Enables specific binding to the target antigen, ensuring accurate recognition of pathogens or diseased cells.
·Fc region (Fragment crystallizable): Binds to Fc receptors on immune cells, mediating mechanisms such as ADCC (Antibody-Dependent Cell-Mediated Cytotoxicity), CDC (Complement-Dependent Cytotoxicity), and phagocytosis. The Fc region also influences pharmacokinetics, half-life, and immunogenicity.
Complementarity-Determining Regions (CDRs) are the most variable segments of the antibody's variable region, comprising six loop structures (CDR1–CDR3 from both heavy and light chains). Among these, CDR3 exhibits the highest sequence diversity and is crucial for determining target specificity. Rational selection or artificial optimization of the CDRs can greatly improve binding affinity and selectivity, enhancing clinical efficacy.
Antibody diversity stems from both the complexity of the natural immune system and advances in bioengineering. From murine monoclonal antibodies to humanized and fully human antibodies, antibody fragments (e.g., scFv, Fab), and more recently, bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs), the field has evolved significantly. These innovations have helped overcome challenges such as high immunogenicity, limited tissue penetration, and narrow therapeutic effects.
With deeper insights into structure-function relationships and the application of cutting-edge technologies such as AI-assisted design and synthetic biology, future antibody therapeutics are expected to become more precise and efficient—paving the way for personalized medicine and next-generation therapies.
Monoclonal Antibodies (mAbs)
Monoclonal antibodies (mAbs) are among the most representative therapeutic proteins in modern biopharmaceuticals, known for their high specificity, strong affinity, and well-defined mechanisms of action. They are widely used in the treatment of cancers, autoimmune diseases, infectious diseases, and cardiovascular disorders. The core feature of mAbs lies in their ability to precisely recognize and bind to specific antigenic targets, enabling them to neutralize toxins, block signaling pathways, or activate immune responses. With continuous technological advancements, monoclonal antibodies have evolved from murine and chimeric formats to fully humanized versions, significantly reducing immunogenicity and improving clinical efficacy.
However, the development of monoclonal antibodies is inherently complex. First, target selection and validation remain challenging; identifying clinically valuable targets from a vast pool of candidates requires extensive experimental support. Second, antibody screening is time-consuming and costly—traditional hybridoma techniques or phage display libraries often take months or more to yield viable antibody candidates. Third, the processes of affinity maturation and humanization are labor-intensive, involving multiple rounds of mutagenesis, screening, and functional assessment. Additionally, issues such as suboptimal pharmacokinetics, low expression levels, and immunogenicity risks often limit the development efficiency and druggability of monoclonal antibodies.
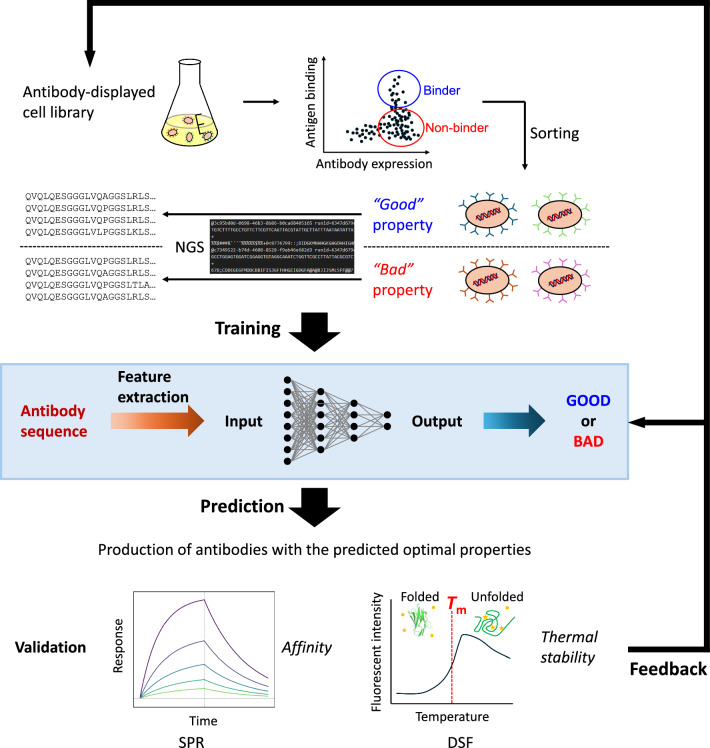
With the evolution of technology, artificial intelligence is becoming a key driving force in monoclonal antibody development. By training deep learning models on large-scale antibody-antigen interaction datasets, AI can efficiently predict antigen epitopes, simulate antibody-binding conformations, and rapidly generate candidate antibody sequences with high affinity and stability. Furthermore, AI can assist with humanization strategies, optimize CDR (complementarity-determining region) structures, and predict ADMET properties (absorption, distribution, metabolism, excretion, and toxicity), thereby significantly improving R&D efficiency and success rates, while shortening the timeline from discovery to preclinical development.
Eureka LS, with its robust multimodal data integration capabilities, offers a new intelligent solution for the design and optimization of monoclonal antibodies. In critical development stages—such as antibody-antigen pairing, epitope mapping, sequence characterization, target selection, and affinity profiling—Eureka LS systematically integrates heterogeneous data from multiple sources. Combined with deep learning algorithms, the platform builds a comprehensive evaluation framework for antibody properties, helping researchers efficiently identify high-potential candidate molecules with strong specificity, binding strength, and drug-like profiles. This intelligent analytics workflow enables early-stage identification of promising antibodies, significantly enhancing development efficiency and reducing the risk of failure.
On top of that, Eureka LS is backed by the high-value Synapse database, which includes approved drug and patent data. The platform offers in-depth analyses of marketed antibody drugs, including therapeutic performance, indication landscape, IP status, and market trends, providing end-to-end decision-making support for project initiation, licensing, technology transfer, and biosimilar development. Eureka LS not only helps companies mitigate IP risks and seize market windows but also allows users to configure AI modules based on specific R&D needs, with customizable analysis parameters, focus areas, and report formats. This highly integrated and configurable data-driven approach significantly boosts operational efficiency and enables biopharma companies to maintain innovation leadership and a competitive edge in an increasingly dynamic industry.
Bispecific Antibodies (BsAbs)
Bispecific antibodies (BsAbs) are genetically engineered antibody molecules capable of simultaneously binding two different antigens or two distinct epitopes on the same antigen, thereby overcoming the limitation of traditional monoclonal antibodies that can only target a single epitope. Designed to integrate multiple biological functions, BsAbs can, for instance, bridge T cells and tumor cells to activate the immune system (e.g., CD3 × tumor antigen bispecifics), block two key signaling pathways simultaneously to prevent tumor immune escape (e.g., EGFR × MET bispecifics), or enhance tissue penetration and target selectivity, thereby improving therapeutic efficacy while reducing off-target toxicity.
BsAbs come in diverse structural formats, including IgG-based bispecific antibodies, tandem scFv constructs such as BiTEs (Bispecific T-cell Engagers), and engineered heterodimer formats like CrossMab using "Knobs-into-Holes" technology. Each format has unique structural and functional features tailored to specific therapeutic needs and application scenarios.
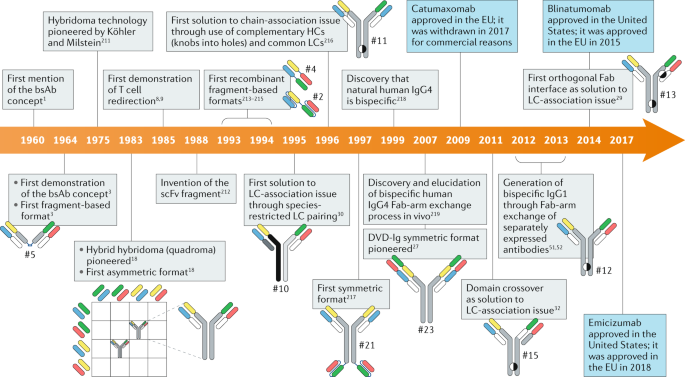
However, the development of BsAbs faces significant challenges. Structurally, one of the central issues is how to spatially organize two distinct antigen-binding domains within a constrained framework without causing steric hindrance that impairs functionality. Chain mispairing is another major bottleneck, especially in formats requiring co-expression of two different heavy and light chains, which can result in non-functional heterodimers or unwanted homodimers, thereby complicating purification and increasing manufacturing costs. Furthermore, pharmacokinetic profiles can be suboptimal—some BsAbs exhibit accelerated clearance and shortened half-lives due to altered structural properties, compromising therapeutic durability.
Immunotoxicity and safety also pose critical concerns, particularly with T cell-engaging BsAbs that may induce excessive immune activation, leading to cytokine release syndrome (CRS) or neurotoxicity. Additionally, complex manufacturing processes and the lack of standardized quality control benchmarks introduce uncertainties in the transition from lab-scale research to large-scale industrial production. As such, leveraging AI-assisted design, novel expression systems, and high-throughput screening platforms has become essential to optimizing BsAb development workflows and accelerating their clinical translation and broader application.
Given the molecular structural complexity, target combination variability, and functional prediction challenges, traditional trial-and-error approaches and experience-driven methods are increasingly inadequate for the efficient and high-quality development of next-generation BsAbs. Artificial intelligence, using deep learning, protein structure prediction, binding affinity modeling, and multi-objective optimization algorithms, can mine large-scale biological datasets to identify promising target combinations and assist in the design of BsAb candidates with optimal spatial conformation and functional activity. This dramatically improves the efficiency and success rate of candidate screening and accelerates the progression from proof-of-concept to preclinical development.
Eureka LS utilizes advanced algorithms to analyze the structures and sequences of approved bispecific antibodies, helping researchers identify optimal combinations of antigen-binding domains from extensive specialized datasets. By learning from existing structural and sequence information, the platform can intelligently predict spatial arrangements of binding domains and propose rational antibody designs that minimize steric hindrance, enhancing the stability and activity of BsAb molecules.
Moreover, Eureka LS plays a critical role in sequence optimization, immunogenicity assessment, and preclinical simulation for bispecific antibodies. Drawing on comprehensive antibody development data—including antibody-antigen pairings, epitope mapping, target selection, and binding affinity—Eureka LS systematically screens and predicts the most promising antibody candidates. This intelligent process not only accelerates decision-making but also significantly reduces the risk of downstream failure, enabling researchers to focus early-stage efforts on candidates with the highest likelihood of success. In doing so, the platform enhances both the development efficiency and clinical translation potential of bispecific antibody therapeutics.
Single-Domain Antibodies (VHH/Nb)
Single-domain antibodies (sdAbs), also known as nanobodies (Nbs), are derived from the variable domain of heavy-chain-only antibodies (VHH) naturally found in camelid species. With a molecular weight of approximately 15 kDa—significantly smaller than conventional IgG antibodies (~150 kDa)—nanobodies exhibit excellent tissue penetration, high affinity, and remarkable thermal stability. These characteristics make them particularly suitable for targeting solid tumors and crossing the blood-brain barrier. For example, certain nanobodies have been successfully applied in Alzheimer's disease research, demonstrating promising central nervous system (CNS) permeability.
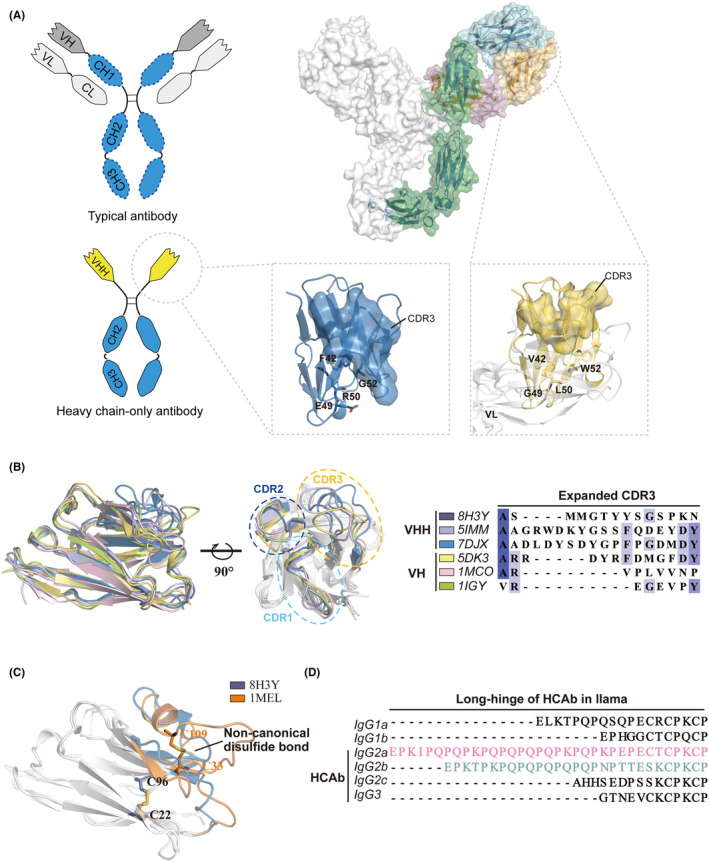
Due to their unique physicochemical properties, single-domain antibodies offer numerous advantages, including high affinity, strong stability, and ease of engineering. However, their structural simplicity also imposes several limitations. First, the absence of an Fc region precludes Fc-mediated functions such as ADCC, CDC, or phagocytosis, which limits their use in immunotherapy strategies that rely on these mechanisms. Second, rapid clearance from the body due to their small size and renal filtration leads to a short half-life, requiring frequent administration or PEGylation to extend circulation time. Third, despite being derived from mammals, VHHs may still pose immunogenicity risks in humans. Lastly, their monovalent nature complicates the formation of multivalent binding formats, necessitating engineering efforts to generate multimeric constructs, which increases development complexity.
Artificial intelligence (AI) significantly enhances the efficiency and success rate of nanobody development by enabling accurate prediction of antigen–antibody binding modes, optimizing CDR structures, and guiding genetic engineering modifications. During the structural design and functional validation stages, Eureka LS employs advanced multimodal AI algorithms to perform high-precision predictions for nanobodies. By analyzing the spatial arrangements of known antigen-binding domains, the platform helps avoid functional failures due to steric hindrance, accelerates decision-making, and reduces downstream risk. Eureka LS learns from large-scale datasets of known antibody-antigen complexes to identify the most optimal VH/VL pairings found in approved drugs. This structure-based rational design approach significantly reduces the number of required trial-and-error experiments and lays a strong foundation for subsequent development.
Antibody–Cytokine Fusion Proteins
Antibody–cytokine fusion proteins are engineered molecules that combine an antibody with an immunostimulatory cytokine (e.g., IL-2, IL-12, IFN-α) to localize cytokine activity and enhance anti-tumor or anti-infective effects while minimizing systemic toxicity. For instance, IL-12 antibody fusion proteins can selectively release IL-12 within the tumor microenvironment, activating T cells and NK cells to boost immune responses.

Despite their theoretical potential, antibody–cytokine fusion proteins face several development challenges. Firstly, structural instability may lead to degradation or reduced biological activity. Secondly, maintaining cytokine activity without compromising antibody targeting is complex, as fusion may mask functional domains. Moreover, while local delivery mitigates systemic toxicity, there remains a risk of excessive immune activation—particularly cytokine release syndrome (CRS)—especially in heterogeneous patient populations. Finally, the manufacturing and quality control of these fusion proteins are technically demanding due to difficulties in maintaining fusion consistency, posing additional challenges for industrial production and regulatory compliance.
AI has made breakthrough advances in protein design, including the creation of "non-natural" antibodies that are not derived from the traditional immune repertoire. For example, David Baker’s team utilized the RFdiffusion algorithm to design RIBs—high-specificity binders targeting disordered regions of the Ras oncoprotein’s C-terminus—offering new therapeutic strategies where conventional antibodies fail to bind highly polar and dynamic regions.
With Eureka LS, researchers can deeply integrate AI into the screening and selection process for antibody–cytokine fusion proteins. The platform analyzes antibody–antigen pairing data, epitope characteristics, and other key parameters to identify and rank the most promising candidates from vast libraries. By deeply embedding AI into the workflow, Eureka LS enhances the efficiency of fusion protein development and provides a robust technological foundation for the creation of safer, more controllable, and personalized immunotherapies.
Conclusion
The structural innovation of next-generation antibody therapeutics is rapidly expanding their applications across disease areas. From classical monoclonal antibodies to highly engineered bispecific antibodies, single-domain antibodies, and antibody–cytokine fusion proteins, each format offers unique advantages in specific therapeutic contexts. However, these sophisticated structures also bring a series of development challenges—ranging from difficult target selection and unstable conformations to complex purification, unpredictable pharmacokinetics, and toxicity risks.
Artificial intelligence is emerging as a transformative force in antibody drug development. Through deep learning, protein language models, and structure prediction tools, AI enables comprehensive optimization across antibody sequence, structure, function, and safety dimensions—significantly shortening development cycles and improving success rates.
As a leading platform in this transformation, Eureka LS provides end-to-end support for antibody drug discovery, optimization, and preclinical evaluation. By integrating intelligent algorithms that assist users in screening and predicting antibody candidates, Eureka LS helps identify the most promising molecules for development—paving the way for innovative antibody therapies to progress efficiently from bench to bedside.
Discover Eureka LS: AI Agents Built for Biopharma Efficiency
Stop wasting time on biopharma busywork. Meet Eureka LS - your AI agent squad for drug discovery.
▶ See how 50+ research teams saved 300+ hours/month
From reducing screening time to simplifying Markush drafting, our AI Agents are ready to deliver immediate value. Explore Eureka LS today and unlock powerful capabilities that help you innovate with confidence.
Reference
- 1.Lodge J, Kajtar L, Duxbury R, Hall D, Burley GA, Cordy J, Yates JWT, Rattray Z. Quantifying antibody binding: techniques and therapeutic implications. MAbs. 2025 Dec;17(1):2459795. doi: 10.1080/19420862.2025.2459795.
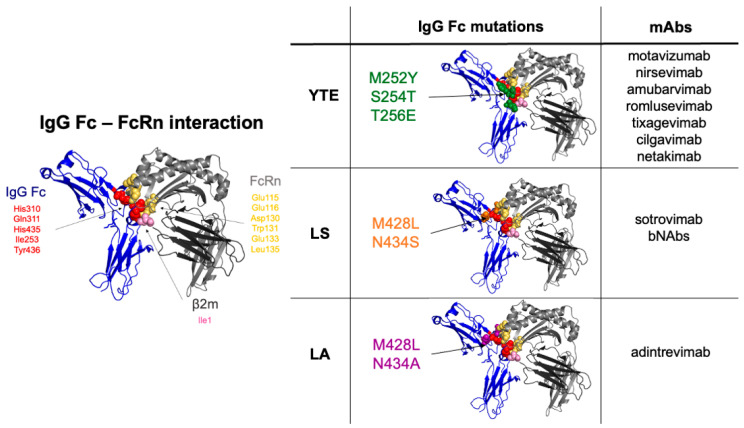
- 2.Ramdani Y, Lamamy J, Watier H, Gouilleux-Gruart V. Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review. Int J Mol Sci. 2022 Aug 24;23(17):9604. doi: 10.3390/ijms23179604.
- 3.Matsunaga R, Tsumoto K. Accelerating antibody discovery and optimization with high-throughput experimentation and machine learning. J Biomed Sci. 2025 May 9;32(1):46. doi: 10.1186/s12929-025-01141-x.
- 4.Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608. doi: 10.1038/s41573-019-0028-1.
- 5.Yu T, Zheng F, He W, Muyldermans S, Wen Y. Single domain antibody: Development and application in biotechnology and biopharma. Immunol Rev. 2024 Nov;328(1):98-112. doi: 10.1111/imr.13381
- 6.Hutmacher C, Neri D. Antibody-cytokine fusion proteins: Biopharmaceuticals with immunomodulatory properties for cancer therapy. Adv Drug Deliv Rev. 2019 Feb 15;141:67-91. doi: 10.1016/j.addr.2018.09.002.