Developing Hypertonic Formulations for Innovative Medical Devices
Hypertonic Formulation Background and Objectives
Hypertonic formulations have emerged as a critical area of research and development in the field of innovative medical devices. These formulations, characterized by their higher solute concentration compared to physiological fluids, have shown remarkable potential in various therapeutic applications. The evolution of hypertonic formulations can be traced back to the early 20th century, with significant advancements occurring in recent decades due to improved understanding of osmotic principles and their physiological effects.
The primary objective of developing hypertonic formulations for medical devices is to leverage osmotic gradients to achieve specific therapeutic outcomes. These formulations can be utilized in diverse applications, including wound healing, drug delivery, and tissue engineering. By creating an osmotic differential, hypertonic formulations can facilitate fluid movement, enhance drug penetration, and modulate cellular responses in targeted tissues.
Recent technological advancements have expanded the scope of hypertonic formulations, enabling the development of smart medical devices that can respond to physiological changes. This has opened up new possibilities in personalized medicine and targeted therapies. The integration of hypertonic formulations with innovative delivery systems and biomaterials has further enhanced their efficacy and versatility in medical applications.
One of the key trends in this field is the development of multi-functional hypertonic formulations that can simultaneously address multiple therapeutic needs. These advanced formulations often incorporate bioactive compounds, nanoparticles, or stimuli-responsive elements to achieve synergistic effects. The goal is to create intelligent medical devices that can adapt to the dynamic physiological environment and provide optimal therapeutic outcomes.
The objectives of current research in hypertonic formulations for medical devices are multifaceted. Scientists and engineers are focusing on enhancing the stability and biocompatibility of these formulations to ensure their safety and efficacy in long-term applications. Additionally, there is a growing emphasis on developing eco-friendly and sustainable hypertonic formulations to align with global environmental concerns.
Another crucial objective is to optimize the controlled release and targeted delivery of therapeutic agents using hypertonic formulations. This involves designing sophisticated delivery systems that can maintain the desired osmotic gradient over extended periods while ensuring precise spatial and temporal control of drug release. Such advancements could revolutionize the treatment of chronic conditions and improve patient compliance.
As the field progresses, researchers are also exploring the potential of combining hypertonic formulations with emerging technologies such as 3D printing and microfluidics. These integrations could lead to the development of highly customized medical devices tailored to individual patient needs, marking a significant step towards precision medicine.
Market Analysis for Hypertonic Medical Devices
The market for hypertonic medical devices is experiencing significant growth, driven by increasing prevalence of chronic diseases, rising geriatric population, and advancements in healthcare technologies. Hypertonic formulations, characterized by higher solute concentration compared to physiological fluids, are finding diverse applications in medical devices across various therapeutic areas.
The global hypertonic medical devices market is segmented based on product types, including hypertonic saline solutions, hypertonic dextrose solutions, and other specialized formulations. These products are utilized in wound care, respiratory therapy, oncology, and other medical fields. The wound care segment, particularly for chronic wound management, represents a substantial market share due to the rising incidence of diabetic foot ulcers and pressure sores.
Geographically, North America dominates the hypertonic medical devices market, followed by Europe and Asia-Pacific. The United States, with its advanced healthcare infrastructure and high healthcare expenditure, leads in market adoption. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth due to improving healthcare access and increasing awareness about advanced medical technologies.
Key market drivers include the growing burden of chronic respiratory diseases, such as cystic fibrosis and bronchiectasis, where hypertonic saline solutions are used for airway clearance. The oncology sector also contributes significantly to market growth, with hypertonic solutions being employed in certain cancer treatments and supportive care.
The competitive landscape of the hypertonic medical devices market is characterized by the presence of both established pharmaceutical companies and innovative medical device manufacturers. Major players are focusing on product innovations, strategic collaborations, and geographical expansions to strengthen their market positions.
Challenges in the market include regulatory hurdles for new product approvals and the need for clinical evidence supporting the efficacy of hypertonic formulations in various applications. Additionally, the market faces competition from alternative treatment modalities and the potential for side effects associated with hypertonic solutions in certain patient populations.
Future market trends point towards the development of novel hypertonic formulations with enhanced therapeutic properties and improved delivery mechanisms. There is also a growing interest in combining hypertonic solutions with other active ingredients to create multifunctional medical devices, particularly in wound care and drug delivery applications.
Current Challenges in Hypertonic Formulation Development
The development of hypertonic formulations for innovative medical devices faces several significant challenges that researchers and manufacturers must overcome. One of the primary obstacles is achieving and maintaining the desired osmolality in the formulation. Hypertonic solutions require a delicate balance of solutes to create an osmotic gradient, which is crucial for their therapeutic effects. However, ensuring consistent osmolality throughout the product's shelf life can be problematic due to potential degradation or interaction of components over time.
Another challenge lies in the selection of appropriate excipients that can withstand high osmotic pressures without compromising the stability or efficacy of the active ingredients. Many traditional excipients may not be suitable for hypertonic formulations, necessitating the exploration of novel stabilizers, buffers, and preservatives that can function effectively in high-solute environments.
The viscosity of hypertonic formulations presents an additional hurdle, particularly in the context of medical devices. Highly concentrated solutions often exhibit increased viscosity, which can impact the flow characteristics and delivery mechanisms of devices such as infusion pumps or nebulizers. Balancing the need for high solute concentration with acceptable rheological properties is a complex task that requires extensive formulation expertise.
Compatibility issues between the hypertonic formulation and device materials pose another significant challenge. The high osmotic pressure and potential corrosiveness of some hypertonic solutions can lead to degradation of device components, potentially compromising the integrity of the medical device or altering the formulation's properties during storage or administration.
Furthermore, the potential for tissue irritation or damage at the site of administration is a critical concern for hypertonic formulations. The development process must carefully consider the physiological impact of high osmolality on biological tissues and design formulations that minimize adverse effects while maintaining therapeutic efficacy.
Regulatory compliance adds another layer of complexity to hypertonic formulation development. Demonstrating the safety and efficacy of these specialized formulations often requires extensive stability studies, biocompatibility testing, and clinical trials. Meeting regulatory standards for both the formulation and the associated medical device can be a time-consuming and resource-intensive process.
Lastly, scale-up and manufacturing of hypertonic formulations present unique challenges. Ensuring homogeneity and preventing precipitation or crystallization during large-scale production requires specialized equipment and processes. Additionally, maintaining sterility in high-osmolality environments may necessitate the development of novel sterilization techniques or packaging solutions.
Existing Hypertonic Formulation Technologies
01 Hypertonic solutions for medical applications
Hypertonic formulations are used in various medical applications, including wound healing, reducing edema, and treating dehydration. These solutions have a higher solute concentration than body fluids, which creates an osmotic gradient that can draw fluid out of tissues or promote fluid absorption, depending on the application.- Hypertonic solutions for medical applications: Hypertonic formulations are used in various medical applications, including wound healing, osmotic therapy for cerebral edema, and treatment of cystic fibrosis. These solutions have a higher solute concentration than body fluids, creating an osmotic gradient that can draw fluid out of tissues or promote hydration in specific areas.
- Hypertonic formulations for ophthalmic use: Hypertonic solutions are developed for ophthalmic applications, such as treating dry eye syndrome and reducing corneal edema. These formulations typically contain high concentrations of osmotically active agents like sodium chloride or glycerin to draw excess fluid from the cornea and relieve symptoms associated with various eye conditions.
- Hypertonic formulations in cell culture and preservation: Hypertonic solutions play a crucial role in cell culture techniques and preservation of biological materials. These formulations are used to create osmotic stress in cells, study cellular responses, and optimize cryopreservation protocols for various cell types and tissues.
- Hypertonic formulations for nasal and respiratory applications: Hypertonic saline solutions are formulated for nasal and respiratory applications, including treatment of chronic sinusitis, allergic rhinitis, and improving mucociliary clearance in patients with respiratory conditions. These formulations help reduce inflammation and improve breathing by drawing fluid from the mucous membranes.
- Hypertonic formulations in sports and exercise recovery: Hypertonic solutions are developed for use in sports and exercise recovery, focusing on rapid rehydration and electrolyte replenishment. These formulations typically contain higher concentrations of carbohydrates and electrolytes compared to isotonic sports drinks, aiming to accelerate fluid absorption and restore energy levels post-exercise.
02 Hypertonic formulations for ophthalmic use
Hypertonic solutions are developed for ophthalmic applications, such as treating dry eye syndrome or reducing corneal edema. These formulations typically contain high concentrations of osmotically active agents that help draw excess fluid from the cornea or promote tear production.Expand Specific Solutions03 Hypertonic formulations for nasal and respiratory applications
Hypertonic solutions are used in nasal sprays and nebulizers to treat respiratory conditions. These formulations can help reduce inflammation, improve mucociliary clearance, and alleviate congestion in conditions such as sinusitis, allergic rhinitis, and cystic fibrosis.Expand Specific Solutions04 Hypertonic formulations for cell preservation and cryoprotection
Hypertonic solutions are utilized in cell preservation techniques and cryoprotection. These formulations help maintain cell viability during freezing and thawing processes by controlling osmotic stress and preventing ice crystal formation.Expand Specific Solutions05 Hypertonic formulations for oral rehydration therapy
Hypertonic oral rehydration solutions are developed for treating dehydration, particularly in cases of diarrheal diseases. These formulations typically contain a balanced mixture of electrolytes and carbohydrates to promote rapid fluid absorption in the intestines.Expand Specific Solutions
Key Players in Hypertonic Formulation Industry
The development of hypertonic formulations for innovative medical devices is in a growth phase, with increasing market size and technological advancements. The global market for such formulations is expanding due to rising demand in various medical applications. Technological maturity varies among key players, with established pharmaceutical companies like Novartis AG, Merck Patent GmbH, and Eli Lilly & Co. leading in research and development. Emerging biotech firms such as Rigel Pharmaceuticals and Frequency Therapeutics are also making significant contributions. The competitive landscape is characterized by a mix of large pharmaceutical corporations and specialized biotech companies, each leveraging their unique strengths in formulation development and device integration.
Novartis AG
Eli Lilly & Co.
Innovative Approaches in Hypertonic Solution Design
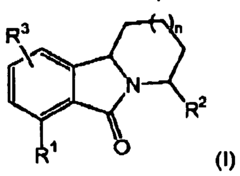
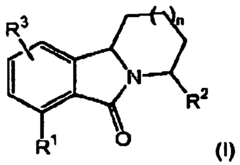
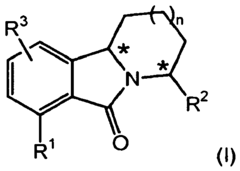
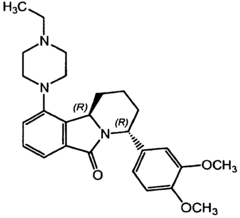
- Development of novel tricyclic compounds that act as urotensin-II receptor antagonists, specifically compounds of Formula (I), which are used in pharmaceutical compositions to treat urotensin-II mediated disorders, including vascular hypertension, heart failure, and renal failure.
- The use of a hypertonic liquid composition comprising specific concentrations of sodium chloride and hydroxyethyl starch, administered perioperatively, reduces inflammatory reactions and promotes granulation tissue formation, thereby accelerating wound healing.
Regulatory Framework for Hypertonic Medical Devices
The regulatory framework for hypertonic medical devices is a complex and evolving landscape that manufacturers must navigate to ensure compliance and market access. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing these devices. The FDA classifies medical devices into three categories based on their risk level and intended use, with hypertonic formulations typically falling under Class II or III, requiring more stringent controls.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance, and safety. Class III devices, considered high-risk, require a more rigorous Premarket Approval (PMA) process, including clinical trials to demonstrate safety and efficacy.
In the European Union, the regulatory landscape has recently undergone significant changes with the implementation of the Medical Device Regulation (MDR) in 2021. This new regulation has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers of hypertonic medical devices must now comply with these enhanced regulations, which include a new risk classification system and stricter requirements for clinical evaluation.
The regulatory framework also encompasses quality management systems, such as ISO 13485, which is specifically designed for medical device manufacturers. Compliance with this standard is often a prerequisite for regulatory approval in many jurisdictions. Additionally, manufacturers must adhere to Good Manufacturing Practices (GMP) to ensure consistent product quality and safety.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to monitor the performance and safety of their devices after market introduction, reporting any adverse events or malfunctions to the relevant authorities. This ongoing vigilance helps to identify and address potential issues promptly, ensuring patient safety and product efficacy.
Internationally, the Global Harmonization Task Force (GHTF) and its successor, the International Medical Device Regulators Forum (IMDRF), have been working towards harmonizing regulatory requirements across different countries. These efforts aim to streamline the approval process and reduce barriers to global market access for innovative medical devices, including those utilizing hypertonic formulations.
As the field of hypertonic medical devices continues to advance, regulatory bodies are adapting their frameworks to keep pace with technological innovations. This includes developing guidance documents for novel technologies and refining regulations to address emerging safety and efficacy concerns specific to hypertonic formulations. Manufacturers must stay informed about these evolving requirements to ensure ongoing compliance and successful market entry for their innovative products.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in developing hypertonic formulations for innovative medical devices. These formulations, characterized by higher solute concentrations than physiological fluids, must be carefully designed to minimize potential adverse effects on biological systems.
The primary concern in hypertonic formulations is osmotic stress on cells and tissues. Excessive osmotic pressure can lead to cellular dehydration, shrinkage, and potential damage to cell membranes. To mitigate these risks, developers must carefully balance the osmolarity of the formulation with its intended therapeutic effect. This often involves extensive in vitro testing using relevant cell lines to assess cellular responses under various osmotic conditions.
Material selection plays a crucial role in ensuring biocompatibility. All components of the hypertonic formulation, including the active ingredients, excipients, and any preservatives, must be thoroughly evaluated for their individual and combined effects on biological systems. This includes assessing potential cytotoxicity, immunogenicity, and long-term tissue compatibility. Developers often rely on established biocompatibility standards, such as ISO 10993, to guide their testing protocols and ensure comprehensive safety evaluations.
The interaction between the hypertonic formulation and the medical device itself must also be considered. Materials used in device construction should be resistant to degradation or leaching when exposed to the hypertonic environment. This is particularly important for implantable or long-term use devices, where prolonged contact between the formulation and device materials could lead to unintended chemical reactions or material breakdown.
Sterility and microbial control are critical aspects of safety for hypertonic formulations. The high solute concentration can create an environment that inhibits microbial growth, but proper sterilization techniques and preservative systems may still be necessary, depending on the specific application and storage conditions. Developers must carefully balance the need for microbial control with potential impacts on biocompatibility and efficacy.
Regulatory considerations are integral to the development process. Hypertonic formulations for medical devices often fall under stringent regulatory scrutiny, requiring extensive safety data and clinical evidence before approval. This may include biocompatibility testing, toxicology studies, and clinical trials to demonstrate both safety and efficacy. Developers must engage with regulatory bodies early in the development process to ensure compliance with all relevant guidelines and standards.
Long-term safety monitoring is essential for hypertonic formulations, particularly those intended for chronic use. Post-market surveillance and ongoing safety studies can help identify any unforeseen biocompatibility issues or long-term effects that may not have been apparent during initial development and testing phases. This continuous monitoring process is crucial for maintaining the safety profile of the product throughout its lifecycle.