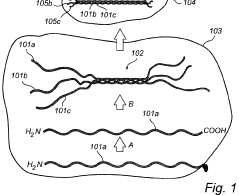
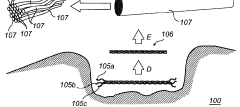
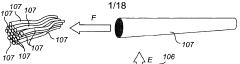
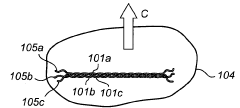
Enhancing Kevlar’s Biocompatibility for Medical Implants
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kevlar Biocompatibility Background and Objectives
Kevlar, a high-strength synthetic fiber developed by DuPont in the 1960s, has long been renowned for its exceptional mechanical properties. Initially designed for use in automotive tires, Kevlar's applications have expanded to include bulletproof vests, aerospace components, and sporting goods. However, its potential in the medical field, particularly for implants, has only recently begun to be explored.
The journey of Kevlar in medical applications started with the recognition of its unique combination of strength, lightweight nature, and chemical stability. These properties make it an attractive candidate for various medical implants, where durability and biocompatibility are crucial. The primary challenge, however, lies in enhancing Kevlar's biocompatibility to ensure its safe and effective use within the human body.
Biocompatibility refers to the ability of a material to perform with an appropriate host response in a specific application. For Kevlar, this means modifying its surface properties to prevent adverse reactions such as inflammation, rejection, or encapsulation when implanted in biological tissues. The goal is to create a Kevlar-based material that not only maintains its superior mechanical properties but also integrates seamlessly with the surrounding biological environment.
The objectives of enhancing Kevlar's biocompatibility for medical implants are multifaceted. Firstly, researchers aim to develop surface modification techniques that can alter Kevlar's chemical and physical properties without compromising its structural integrity. This includes exploring various coating methods, chemical treatments, and nanostructure modifications to improve cell adhesion and proliferation on Kevlar surfaces.
Secondly, there is a focus on understanding and mitigating the body's immune response to Kevlar implants. This involves studying the interaction between Kevlar and various biological components, such as proteins, cells, and tissues, to design strategies that minimize foreign body reactions and promote tissue integration.
Another critical objective is to enhance the long-term stability and performance of Kevlar-based implants in physiological conditions. This includes investigating the material's degradation behavior, if any, and developing methods to control or prevent such degradation to ensure the implant's longevity and functionality.
Furthermore, researchers are exploring ways to functionalize Kevlar surfaces to impart additional beneficial properties, such as antimicrobial activity or drug delivery capabilities. This could potentially expand the range of medical applications for Kevlar-based implants, from orthopedics to cardiovascular devices.
The ultimate goal of this research is to position Kevlar as a viable and superior alternative to existing biomaterials in certain medical implant applications. By addressing the biocompatibility challenges, researchers hope to leverage Kevlar's exceptional mechanical properties to create implants that offer improved performance, durability, and patient outcomes compared to current options.
The journey of Kevlar in medical applications started with the recognition of its unique combination of strength, lightweight nature, and chemical stability. These properties make it an attractive candidate for various medical implants, where durability and biocompatibility are crucial. The primary challenge, however, lies in enhancing Kevlar's biocompatibility to ensure its safe and effective use within the human body.
Biocompatibility refers to the ability of a material to perform with an appropriate host response in a specific application. For Kevlar, this means modifying its surface properties to prevent adverse reactions such as inflammation, rejection, or encapsulation when implanted in biological tissues. The goal is to create a Kevlar-based material that not only maintains its superior mechanical properties but also integrates seamlessly with the surrounding biological environment.
The objectives of enhancing Kevlar's biocompatibility for medical implants are multifaceted. Firstly, researchers aim to develop surface modification techniques that can alter Kevlar's chemical and physical properties without compromising its structural integrity. This includes exploring various coating methods, chemical treatments, and nanostructure modifications to improve cell adhesion and proliferation on Kevlar surfaces.
Secondly, there is a focus on understanding and mitigating the body's immune response to Kevlar implants. This involves studying the interaction between Kevlar and various biological components, such as proteins, cells, and tissues, to design strategies that minimize foreign body reactions and promote tissue integration.
Another critical objective is to enhance the long-term stability and performance of Kevlar-based implants in physiological conditions. This includes investigating the material's degradation behavior, if any, and developing methods to control or prevent such degradation to ensure the implant's longevity and functionality.
Furthermore, researchers are exploring ways to functionalize Kevlar surfaces to impart additional beneficial properties, such as antimicrobial activity or drug delivery capabilities. This could potentially expand the range of medical applications for Kevlar-based implants, from orthopedics to cardiovascular devices.
The ultimate goal of this research is to position Kevlar as a viable and superior alternative to existing biomaterials in certain medical implant applications. By addressing the biocompatibility challenges, researchers hope to leverage Kevlar's exceptional mechanical properties to create implants that offer improved performance, durability, and patient outcomes compared to current options.
Medical Implant Market Analysis
The medical implant market has been experiencing significant growth and transformation in recent years, driven by technological advancements, an aging population, and increasing prevalence of chronic diseases. This market encompasses a wide range of devices, including orthopedic implants, cardiovascular implants, dental implants, and neurostimulators, among others. The global medical implant market size was valued at approximately $94 billion in 2020 and is projected to reach $147 billion by 2027, growing at a compound annual growth rate (CAGR) of 7.2% during the forecast period.
Several factors contribute to the expanding demand for medical implants. The rising geriatric population, particularly in developed countries, is a primary driver as older individuals are more prone to degenerative diseases and require implants for improved quality of life. Additionally, the increasing incidence of chronic diseases, such as cardiovascular disorders and osteoarthritis, fuels the need for implantable medical devices. Technological advancements in materials science and bioengineering have led to the development of more durable, biocompatible, and functional implants, further stimulating market growth.
The orthopedic implant segment dominates the market, accounting for the largest share due to the high prevalence of musculoskeletal disorders and the growing number of sports-related injuries. Cardiovascular implants follow closely, driven by the rising incidence of heart diseases and the adoption of minimally invasive procedures. Dental implants also represent a significant portion of the market, propelled by increasing dental awareness and the desire for aesthetic improvements.
Geographically, North America holds the largest market share, attributed to advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative technologies. Europe follows as the second-largest market, while the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare access, rising disposable incomes, and a large patient pool.
The competitive landscape of the medical implant market is characterized by the presence of several major players, including Medtronic, Johnson & Johnson, Stryker Corporation, and Zimmer Biomet. These companies are investing heavily in research and development to introduce novel materials and technologies, such as 3D-printed implants and smart implants with sensing capabilities. The integration of artificial intelligence and machine learning in implant design and manufacturing processes is also emerging as a significant trend, promising to enhance implant performance and patient outcomes.
However, the market faces challenges such as stringent regulatory requirements, high costs associated with implant procedures, and the risk of post-implant complications. The ongoing efforts to enhance biocompatibility and reduce rejection rates are crucial for addressing these challenges and driving further market expansion. In this context, the development of Kevlar-based implants with improved biocompatibility represents a promising avenue for innovation in the medical implant sector.
Several factors contribute to the expanding demand for medical implants. The rising geriatric population, particularly in developed countries, is a primary driver as older individuals are more prone to degenerative diseases and require implants for improved quality of life. Additionally, the increasing incidence of chronic diseases, such as cardiovascular disorders and osteoarthritis, fuels the need for implantable medical devices. Technological advancements in materials science and bioengineering have led to the development of more durable, biocompatible, and functional implants, further stimulating market growth.
The orthopedic implant segment dominates the market, accounting for the largest share due to the high prevalence of musculoskeletal disorders and the growing number of sports-related injuries. Cardiovascular implants follow closely, driven by the rising incidence of heart diseases and the adoption of minimally invasive procedures. Dental implants also represent a significant portion of the market, propelled by increasing dental awareness and the desire for aesthetic improvements.
Geographically, North America holds the largest market share, attributed to advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative technologies. Europe follows as the second-largest market, while the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare access, rising disposable incomes, and a large patient pool.
The competitive landscape of the medical implant market is characterized by the presence of several major players, including Medtronic, Johnson & Johnson, Stryker Corporation, and Zimmer Biomet. These companies are investing heavily in research and development to introduce novel materials and technologies, such as 3D-printed implants and smart implants with sensing capabilities. The integration of artificial intelligence and machine learning in implant design and manufacturing processes is also emerging as a significant trend, promising to enhance implant performance and patient outcomes.
However, the market faces challenges such as stringent regulatory requirements, high costs associated with implant procedures, and the risk of post-implant complications. The ongoing efforts to enhance biocompatibility and reduce rejection rates are crucial for addressing these challenges and driving further market expansion. In this context, the development of Kevlar-based implants with improved biocompatibility represents a promising avenue for innovation in the medical implant sector.
Biocompatible Kevlar Challenges
The development of biocompatible Kevlar for medical implants faces several significant challenges that require innovative solutions. One of the primary obstacles is Kevlar's inherent hydrophobicity, which can lead to poor cell adhesion and integration with surrounding tissues. This characteristic limits its ability to promote tissue growth and healing, essential factors for successful implantation.
Another major challenge lies in modifying Kevlar's surface properties without compromising its exceptional mechanical strength and durability. Any surface treatment or modification must enhance biocompatibility while maintaining the material's structural integrity and performance. This delicate balance is crucial for ensuring the longevity and effectiveness of medical implants.
The potential for inflammatory responses and foreign body reactions presents another hurdle in Kevlar's biomedical applications. The body's immune system may recognize Kevlar as a foreign substance, leading to encapsulation or rejection of the implant. Overcoming this immune response is critical for long-term implant success and patient safety.
Furthermore, the sterilization of Kevlar-based medical devices poses a significant challenge. Traditional sterilization methods, such as high-temperature autoclaving or radiation, may degrade Kevlar's properties. Developing sterilization techniques that are both effective against pathogens and safe for the material's structure is essential for clinical applications.
The controlled degradation or resorption of Kevlar in the body presents another complex challenge. While Kevlar's durability is advantageous in many applications, certain medical implants may require gradual degradation to allow for tissue regeneration or to avoid the need for removal surgeries. Engineering Kevlar to degrade at a controlled rate without producing harmful byproducts is a significant research focus.
Lastly, ensuring consistent quality and reproducibility in the production of biocompatible Kevlar remains a challenge. The manufacturing process must be optimized to produce Kevlar with uniform surface properties and consistent biocompatibility across different batches. This is crucial for regulatory approval and widespread clinical adoption of Kevlar-based medical implants.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, biomedical engineers, and clinicians. Innovative approaches, such as surface functionalization, composite material development, and nanotechnology integration, are being explored to overcome these obstacles and unlock the full potential of Kevlar in medical implant applications.
Another major challenge lies in modifying Kevlar's surface properties without compromising its exceptional mechanical strength and durability. Any surface treatment or modification must enhance biocompatibility while maintaining the material's structural integrity and performance. This delicate balance is crucial for ensuring the longevity and effectiveness of medical implants.
The potential for inflammatory responses and foreign body reactions presents another hurdle in Kevlar's biomedical applications. The body's immune system may recognize Kevlar as a foreign substance, leading to encapsulation or rejection of the implant. Overcoming this immune response is critical for long-term implant success and patient safety.
Furthermore, the sterilization of Kevlar-based medical devices poses a significant challenge. Traditional sterilization methods, such as high-temperature autoclaving or radiation, may degrade Kevlar's properties. Developing sterilization techniques that are both effective against pathogens and safe for the material's structure is essential for clinical applications.
The controlled degradation or resorption of Kevlar in the body presents another complex challenge. While Kevlar's durability is advantageous in many applications, certain medical implants may require gradual degradation to allow for tissue regeneration or to avoid the need for removal surgeries. Engineering Kevlar to degrade at a controlled rate without producing harmful byproducts is a significant research focus.
Lastly, ensuring consistent quality and reproducibility in the production of biocompatible Kevlar remains a challenge. The manufacturing process must be optimized to produce Kevlar with uniform surface properties and consistent biocompatibility across different batches. This is crucial for regulatory approval and widespread clinical adoption of Kevlar-based medical implants.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, biomedical engineers, and clinicians. Innovative approaches, such as surface functionalization, composite material development, and nanotechnology integration, are being explored to overcome these obstacles and unlock the full potential of Kevlar in medical implant applications.
Current Kevlar Biocompatibility Solutions
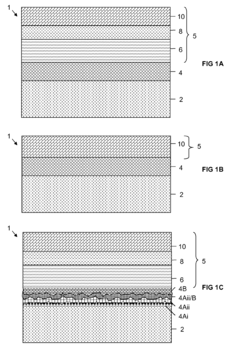
01 Biocompatible Kevlar composites for medical applications
Kevlar fibers are combined with biocompatible materials to create composites suitable for medical applications. These composites maintain Kevlar's strength while improving biocompatibility, making them suitable for implants, prosthetics, and other medical devices that come into contact with biological tissues.- Biocompatible Kevlar composites for medical applications: Kevlar fibers are combined with biocompatible materials to create composites suitable for medical applications. These composites maintain Kevlar's strength while improving biocompatibility, making them suitable for implants, prosthetics, and tissue engineering scaffolds.
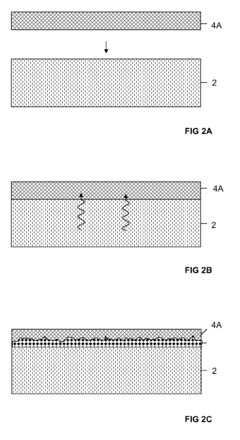
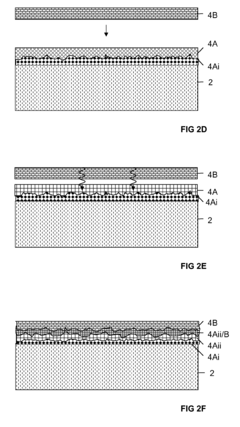
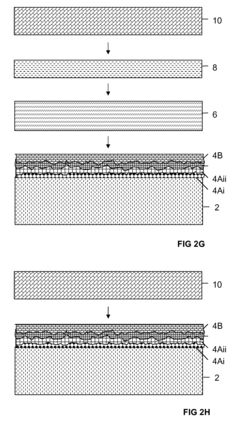
- Surface modification of Kevlar for enhanced biocompatibility: Various surface modification techniques are applied to Kevlar fibers to improve their biocompatibility. These methods include plasma treatment, chemical functionalization, and coating with biocompatible materials, which enhance cell adhesion and reduce foreign body reactions.
- Kevlar-based drug delivery systems: Kevlar fibers are utilized as carriers for drug delivery systems due to their high surface area and stability. The fibers are modified to incorporate and release therapeutic agents in a controlled manner, improving biocompatibility and treatment efficacy.
- Biocompatible Kevlar coatings for medical devices: Specialized coatings are developed to enhance the biocompatibility of Kevlar-based medical devices. These coatings reduce inflammation, prevent bacterial adhesion, and promote integration with surrounding tissues, making Kevlar suitable for long-term implantable devices.
- Kevlar-based tissue engineering scaffolds: Kevlar fibers are incorporated into tissue engineering scaffolds to provide mechanical strength and support cell growth. The scaffolds are designed to mimic natural tissue structures and promote tissue regeneration while maintaining biocompatibility.
02 Surface modification of Kevlar for enhanced biocompatibility
Various surface modification techniques are applied to Kevlar fibers to improve their biocompatibility. These methods include coating with biocompatible polymers, plasma treatment, and chemical functionalization, which can enhance cell adhesion, reduce inflammatory responses, and improve overall integration with biological systems.Expand Specific Solutions03 Kevlar-based scaffolds for tissue engineering
Kevlar fibers are used to create biocompatible scaffolds for tissue engineering applications. These scaffolds provide mechanical support and promote cell growth and tissue regeneration. The unique properties of Kevlar, such as high strength and durability, make it an attractive material for long-term implantable scaffolds.Expand Specific Solutions04 Biocompatible Kevlar nanofibers for drug delivery
Kevlar nanofibers are developed and modified to create biocompatible drug delivery systems. These nanofibers can encapsulate and release drugs in a controlled manner, offering potential applications in wound healing, cancer treatment, and other therapeutic areas where localized drug delivery is beneficial.Expand Specific Solutions05 Evaluation of Kevlar's long-term biocompatibility and biodegradation
Studies are conducted to assess the long-term biocompatibility and potential biodegradation of Kevlar in biological environments. These investigations aim to understand the material's stability, potential toxicity, and interactions with living tissues over extended periods, which is crucial for its use in long-term implantable medical devices.Expand Specific Solutions
Key Players in Biocompatible Materials
The field of enhancing Kevlar's biocompatibility for medical implants is in its early development stage, with significant potential for growth. The market size is expected to expand as the demand for advanced medical implants increases. While the technology is still evolving, several key players are making strides in this area. Companies like Biotronik AG and Orbusneich Medical Pte Ltd. are leveraging their expertise in medical devices to explore Kevlar's applications. Academic institutions such as Zhejiang University and the University of Seville are contributing to fundamental research. The involvement of materials science specialists like SAES Getters SpA and Bharat Forge Ltd. indicates a growing interest in developing innovative biocompatible Kevlar composites for medical use.
Biotronik AG
Technical Solution: Biotronik AG has developed a novel approach to enhance Kevlar's biocompatibility for medical implants, focusing on surface modification techniques. Their method involves plasma treatment of Kevlar fibers to create functional groups that improve cell adhesion and proliferation[1]. This is followed by a coating process using biocompatible polymers such as polyethylene glycol (PEG) or hyaluronic acid, which further enhances the material's integration with surrounding tissues[3]. The company has also explored the incorporation of growth factors and antimicrobial agents into the coating to promote healing and reduce the risk of infection[5]. Biotronik's research has shown promising results in in vitro and animal studies, demonstrating improved cell attachment and reduced inflammatory responses compared to untreated Kevlar[2].
Strengths: Improved cell adhesion and tissue integration, reduced inflammatory response, potential for drug delivery. Weaknesses: Complex manufacturing process, potential long-term stability issues of coatings, higher production costs.
Asahi Kasei Medical Co., Ltd.
Technical Solution: Asahi Kasei Medical has developed a proprietary method for enhancing Kevlar's biocompatibility through a combination of chemical modification and nanostructure engineering. Their approach involves grafting biocompatible polymers onto the Kevlar surface using a controlled radical polymerization technique[4]. This creates a brush-like structure that mimics the extracellular matrix, promoting cell attachment and growth. Additionally, they have incorporated nanoparticles of bioactive ceramics, such as hydroxyapatite, into the polymer coating to enhance osseointegration for orthopedic applications[6]. The company has also developed a method to create microporous structures within the Kevlar fibers, which allows for better tissue ingrowth and vascularization[8]. Preliminary studies have shown improved biocompatibility and reduced foreign body response in soft tissue implants[7].
Strengths: Enhanced cell attachment and tissue integration, improved osseointegration for orthopedic applications, potential for customization based on specific implant requirements. Weaknesses: Complexity of manufacturing process, potential for altered mechanical properties of Kevlar, regulatory challenges due to novel modifications.
Innovative Kevlar Surface Treatments
Designed surfaces for use in medical implants or instruments
PatentInactiveEP2204198A1
Innovation
- A bonding structure with a chromium rich layer and gradient material is deposited between the metal substrate and ceramic coating, providing a smooth transition of mechanical and structural properties to enhance adhesion and prevent cracking, comprising a chromium rich layer and a gradient layer with increasing proportions of intermetallic carbides or nitrides, and an outermost ceramic layer for improved wear resistance.
Collagen coated article
PatentWO2012173555A1
Innovation
- A biocompatible article with a surface comprising collagen fibrils oriented vertically using linker molecules such as poly-L-lysine, which enhances biocompatibility by improving tissue integration and regeneration through a three-dimensional fibril orientation that mimics natural collagen structure.
Regulatory Framework for Medical Implants
The regulatory framework for medical implants plays a crucial role in ensuring the safety and efficacy of devices intended for use within the human body. In the context of enhancing Kevlar's biocompatibility for medical implants, understanding and adhering to these regulations is paramount for successful development and market entry.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical implants through the Center for Devices and Radiological Health (CDRH). The FDA classifies medical devices into three categories based on their risk level, with Class III devices, which include most implants, subject to the most stringent controls. For Kevlar-based implants, manufacturers would likely need to follow the Premarket Approval (PMA) process, which requires extensive clinical trials to demonstrate safety and effectiveness.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern medical implants. These regulations emphasize a life-cycle approach to device safety and mandate rigorous clinical evaluation and post-market surveillance. Kevlar-enhanced implants would need to comply with these regulations, including obtaining CE marking before market entry.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical implants. The approval process involves submitting a premarket application and conducting clinical trials to demonstrate safety and efficacy. The Japanese regulatory framework also emphasizes post-market surveillance and adverse event reporting.
International standards, such as ISO 13485 for quality management systems and ISO 10993 for biological evaluation of medical devices, provide guidelines for manufacturers developing Kevlar-based implants. These standards ensure consistency in quality control and biocompatibility testing across different regulatory jurisdictions.
Regulatory bodies worldwide are increasingly focusing on the long-term safety of implantable devices. For Kevlar-enhanced implants, this means demonstrating not only initial biocompatibility but also long-term stability and safety within the body. Manufacturers must provide comprehensive data on material degradation, potential leaching of components, and long-term tissue response.
As nanotechnology and advanced materials like Kevlar become more prevalent in medical implants, regulatory frameworks are evolving to address these innovations. Agencies are developing new guidance documents and adapting existing regulations to encompass novel materials and their unique properties. This dynamic regulatory landscape requires manufacturers to maintain close communication with regulatory bodies throughout the development process.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical implants through the Center for Devices and Radiological Health (CDRH). The FDA classifies medical devices into three categories based on their risk level, with Class III devices, which include most implants, subject to the most stringent controls. For Kevlar-based implants, manufacturers would likely need to follow the Premarket Approval (PMA) process, which requires extensive clinical trials to demonstrate safety and effectiveness.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern medical implants. These regulations emphasize a life-cycle approach to device safety and mandate rigorous clinical evaluation and post-market surveillance. Kevlar-enhanced implants would need to comply with these regulations, including obtaining CE marking before market entry.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical implants. The approval process involves submitting a premarket application and conducting clinical trials to demonstrate safety and efficacy. The Japanese regulatory framework also emphasizes post-market surveillance and adverse event reporting.
International standards, such as ISO 13485 for quality management systems and ISO 10993 for biological evaluation of medical devices, provide guidelines for manufacturers developing Kevlar-based implants. These standards ensure consistency in quality control and biocompatibility testing across different regulatory jurisdictions.
Regulatory bodies worldwide are increasingly focusing on the long-term safety of implantable devices. For Kevlar-enhanced implants, this means demonstrating not only initial biocompatibility but also long-term stability and safety within the body. Manufacturers must provide comprehensive data on material degradation, potential leaching of components, and long-term tissue response.
As nanotechnology and advanced materials like Kevlar become more prevalent in medical implants, regulatory frameworks are evolving to address these innovations. Agencies are developing new guidance documents and adapting existing regulations to encompass novel materials and their unique properties. This dynamic regulatory landscape requires manufacturers to maintain close communication with regulatory bodies throughout the development process.
Kevlar Implant Safety Testing Protocols
The development of safety testing protocols for Kevlar implants is crucial to ensure their biocompatibility and long-term performance in medical applications. These protocols must be comprehensive, addressing various aspects of implant safety and efficacy.
Initial testing should focus on the material properties of Kevlar-based implants, including tensile strength, elasticity, and durability under physiological conditions. Mechanical stress tests simulating in vivo forces should be conducted to evaluate the implant's ability to withstand long-term use without degradation or failure.
Biocompatibility testing is a critical component of the safety protocols. This includes in vitro cytotoxicity assays to assess the potential harmful effects of Kevlar implants on surrounding tissues. Cell culture experiments using relevant cell lines should be performed to evaluate cell adhesion, proliferation, and potential inflammatory responses.
In vivo studies are essential to understand the long-term effects of Kevlar implants. Animal models should be carefully selected to mimic human physiological conditions. Implantation procedures should be standardized, and animals monitored for extended periods to assess tissue integration, immune responses, and potential complications.
Histological analysis of tissues surrounding the implant is crucial for evaluating local tissue reactions. This should include examination of inflammatory cell infiltration, fibrosis, and potential degradation of the implant material over time.
Systemic effects must also be considered in the safety protocols. Blood tests and organ function analyses should be conducted to detect any potential systemic toxicity or adverse reactions resulting from the implant.
Imaging studies, such as MRI and CT scans, should be included in the protocols to assess the implant's position, integrity, and any potential migration over time. These imaging techniques can also help evaluate tissue integration and identify any abnormal tissue responses.
Biodegradation and wear studies are essential for understanding the long-term stability of Kevlar implants. Simulated physiological environments should be used to assess potential material degradation, and wear particles should be analyzed for their potential biological effects.
Finally, the safety testing protocols should include rigorous statistical analysis of all data collected. This ensures that the results are reliable and can be used to make informed decisions about the safety and efficacy of Kevlar implants for medical applications.
Initial testing should focus on the material properties of Kevlar-based implants, including tensile strength, elasticity, and durability under physiological conditions. Mechanical stress tests simulating in vivo forces should be conducted to evaluate the implant's ability to withstand long-term use without degradation or failure.
Biocompatibility testing is a critical component of the safety protocols. This includes in vitro cytotoxicity assays to assess the potential harmful effects of Kevlar implants on surrounding tissues. Cell culture experiments using relevant cell lines should be performed to evaluate cell adhesion, proliferation, and potential inflammatory responses.
In vivo studies are essential to understand the long-term effects of Kevlar implants. Animal models should be carefully selected to mimic human physiological conditions. Implantation procedures should be standardized, and animals monitored for extended periods to assess tissue integration, immune responses, and potential complications.
Histological analysis of tissues surrounding the implant is crucial for evaluating local tissue reactions. This should include examination of inflammatory cell infiltration, fibrosis, and potential degradation of the implant material over time.
Systemic effects must also be considered in the safety protocols. Blood tests and organ function analyses should be conducted to detect any potential systemic toxicity or adverse reactions resulting from the implant.
Imaging studies, such as MRI and CT scans, should be included in the protocols to assess the implant's position, integrity, and any potential migration over time. These imaging techniques can also help evaluate tissue integration and identify any abnormal tissue responses.
Biodegradation and wear studies are essential for understanding the long-term stability of Kevlar implants. Simulated physiological environments should be used to assess potential material degradation, and wear particles should be analyzed for their potential biological effects.
Finally, the safety testing protocols should include rigorous statistical analysis of all data collected. This ensures that the results are reliable and can be used to make informed decisions about the safety and efficacy of Kevlar implants for medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!