How to Measure Patient Response in Quantum Healing Sessions
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Healing Measurement Background and Objectives
Quantum healing represents a convergence of quantum physics principles with traditional healing practices, aiming to influence health outcomes through quantum-level interactions. The concept emerged in the 1980s, gaining prominence through Deepak Chopra's work, which proposed that consciousness could affect quantum processes in biological systems. Over decades, this field has evolved from theoretical frameworks to experimental applications, with increasing interest in establishing measurable outcomes.
The evolution of quantum healing measurement techniques has progressed from subjective patient reporting to more sophisticated biometric monitoring. Early approaches relied heavily on anecdotal evidence and practitioner observations, lacking standardized metrics. Recent developments incorporate biofield measurement devices, heart rate variability analysis, and electroencephalography to capture physiological responses during quantum healing interventions.
Current research objectives focus on developing reliable, reproducible measurement protocols that can bridge subjective experience with objective physiological markers. This includes establishing baseline parameters for quantum healing responses, identifying key biomarkers that correlate with healing outcomes, and creating standardized assessment tools that maintain sensitivity to subtle energetic changes while meeting scientific rigor requirements.
The technical goals of quantum healing measurement systems include real-time monitoring capabilities, non-invasive data collection methods, and integration with existing medical diagnostic frameworks. Researchers aim to develop instruments capable of detecting quantum-level changes in cellular activity, coherence patterns in biofields, and shifts in consciousness states that may accompany healing responses.
Significant challenges exist in quantifying the often subtle and multidimensional nature of quantum healing effects. Traditional scientific paradigms may not adequately capture the holistic nature of these interventions, necessitating new measurement approaches that can account for interconnected physical, emotional, and consciousness factors.
The field is moving toward multimodal assessment strategies that combine subjective patient-reported outcomes with objective physiological measurements. This integrated approach acknowledges both the measurable biological changes and the experiential dimensions of healing that may not be captured through conventional metrics alone.
Future directions point toward the development of quantum-sensitive biosensors, advanced consciousness mapping technologies, and AI-assisted pattern recognition systems that can identify correlations between quantum interventions and healing responses across diverse patient populations. These technological advances aim to establish quantum healing measurement as a legitimate field of scientific inquiry with practical applications in healthcare settings.
The evolution of quantum healing measurement techniques has progressed from subjective patient reporting to more sophisticated biometric monitoring. Early approaches relied heavily on anecdotal evidence and practitioner observations, lacking standardized metrics. Recent developments incorporate biofield measurement devices, heart rate variability analysis, and electroencephalography to capture physiological responses during quantum healing interventions.
Current research objectives focus on developing reliable, reproducible measurement protocols that can bridge subjective experience with objective physiological markers. This includes establishing baseline parameters for quantum healing responses, identifying key biomarkers that correlate with healing outcomes, and creating standardized assessment tools that maintain sensitivity to subtle energetic changes while meeting scientific rigor requirements.
The technical goals of quantum healing measurement systems include real-time monitoring capabilities, non-invasive data collection methods, and integration with existing medical diagnostic frameworks. Researchers aim to develop instruments capable of detecting quantum-level changes in cellular activity, coherence patterns in biofields, and shifts in consciousness states that may accompany healing responses.
Significant challenges exist in quantifying the often subtle and multidimensional nature of quantum healing effects. Traditional scientific paradigms may not adequately capture the holistic nature of these interventions, necessitating new measurement approaches that can account for interconnected physical, emotional, and consciousness factors.
The field is moving toward multimodal assessment strategies that combine subjective patient-reported outcomes with objective physiological measurements. This integrated approach acknowledges both the measurable biological changes and the experiential dimensions of healing that may not be captured through conventional metrics alone.
Future directions point toward the development of quantum-sensitive biosensors, advanced consciousness mapping technologies, and AI-assisted pattern recognition systems that can identify correlations between quantum interventions and healing responses across diverse patient populations. These technological advances aim to establish quantum healing measurement as a legitimate field of scientific inquiry with practical applications in healthcare settings.
Market Analysis for Quantum Healing Response Metrics
The quantum healing market has witnessed significant growth in recent years, driven by increasing consumer interest in alternative and complementary healthcare approaches. Current market estimates suggest the global alternative medicine sector, which includes quantum healing practices, exceeds $82 billion annually with consistent growth rates between 15-20% in developed markets. Within this broader category, quantum healing response metrics represent an emerging niche with substantial growth potential.
Consumer demand for quantifiable results in quantum healing sessions has created a distinct market opportunity for response measurement technologies and methodologies. Primary market segments include professional practitioners seeking validation tools, healthcare institutions exploring integrative medicine programs, and technology companies developing biometric monitoring devices specifically calibrated for subtle energy interventions.
Regional analysis reveals North America currently dominates the quantum healing metrics market, accounting for approximately 45% of global demand, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. This distribution correlates strongly with disposable income levels and cultural acceptance of complementary medicine approaches.
Market research indicates three primary consumer segments driving demand: established practitioners seeking practice differentiation through scientific validation, healthcare institutions implementing integrative medicine programs requiring outcomes documentation, and individual consumers increasingly demanding evidence of effectiveness for their investment in alternative therapies.
Pricing analysis of current quantum healing response measurement solutions shows significant stratification, with basic biomarker testing kits available from $200-500, mid-range biofeedback equipment priced between $1,500-3,000, and advanced quantum response measurement systems commanding $5,000-15,000. This price segmentation reflects varying levels of technological sophistication and clinical validation.
Competitive landscape assessment reveals approximately 30 companies currently offering quantum healing measurement solutions globally, with market concentration relatively low. The top five providers control only 35% of market share, indicating a fragmented market with opportunities for new entrants with innovative approaches.
Growth projections suggest the quantum healing response metrics market will expand at a compound annual growth rate of 18% over the next five years, outpacing the broader alternative medicine sector. This accelerated growth is attributed to increasing mainstream acceptance, integration with conventional healthcare systems, and technological advancements enabling more precise measurement of previously subjective healing responses.
Consumer demand for quantifiable results in quantum healing sessions has created a distinct market opportunity for response measurement technologies and methodologies. Primary market segments include professional practitioners seeking validation tools, healthcare institutions exploring integrative medicine programs, and technology companies developing biometric monitoring devices specifically calibrated for subtle energy interventions.
Regional analysis reveals North America currently dominates the quantum healing metrics market, accounting for approximately 45% of global demand, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. This distribution correlates strongly with disposable income levels and cultural acceptance of complementary medicine approaches.
Market research indicates three primary consumer segments driving demand: established practitioners seeking practice differentiation through scientific validation, healthcare institutions implementing integrative medicine programs requiring outcomes documentation, and individual consumers increasingly demanding evidence of effectiveness for their investment in alternative therapies.
Pricing analysis of current quantum healing response measurement solutions shows significant stratification, with basic biomarker testing kits available from $200-500, mid-range biofeedback equipment priced between $1,500-3,000, and advanced quantum response measurement systems commanding $5,000-15,000. This price segmentation reflects varying levels of technological sophistication and clinical validation.
Competitive landscape assessment reveals approximately 30 companies currently offering quantum healing measurement solutions globally, with market concentration relatively low. The top five providers control only 35% of market share, indicating a fragmented market with opportunities for new entrants with innovative approaches.
Growth projections suggest the quantum healing response metrics market will expand at a compound annual growth rate of 18% over the next five years, outpacing the broader alternative medicine sector. This accelerated growth is attributed to increasing mainstream acceptance, integration with conventional healthcare systems, and technological advancements enabling more precise measurement of previously subjective healing responses.
Current Challenges in Patient Response Quantification
Quantifying patient responses in quantum healing sessions presents significant methodological challenges due to the intersection of quantum principles with traditional medical assessment frameworks. The subjective nature of healing experiences creates a fundamental barrier to standardized measurement, as patients report widely varying sensations, emotional states, and perceived benefits that resist conventional categorization.
Current biomedical instrumentation lacks specificity for quantum healing phenomena, with most devices designed to measure physiological parameters that may not capture the subtle energetic shifts claimed in quantum healing modalities. While EEG, heart rate variability, and galvanic skin response offer partial insights, these tools were not developed to detect quantum-level biological interactions, creating a technological gap in assessment capabilities.
The placebo effect introduces substantial confounding variables, as the expectation of healing can trigger neurobiological responses that mimic or enhance treatment effects. Researchers struggle to design control protocols that can effectively distinguish between quantum healing mechanisms and placebo responses, particularly when the theoretical framework suggests consciousness itself may influence outcomes.
Reproducibility challenges further complicate measurement efforts, as quantum healing sessions often yield inconsistent results across different practitioners, patients, and environmental contexts. The proposed non-local nature of quantum healing effects suggests that standard controlled experimental designs may be fundamentally incompatible with the phenomena being studied.
Temporal assessment issues arise when determining appropriate measurement intervals, as quantum healing responses reportedly manifest across varying timeframes—from immediate sensations to gradual improvements over weeks or months. This temporal variability complicates before-and-after measurement protocols and challenges traditional clinical trial methodologies.
Interdisciplinary barriers between quantum physics, medicine, psychology, and complementary health fields create conceptual disconnects in measurement approaches. The lack of shared terminology and methodological standards across these disciplines has resulted in fragmented research efforts and inconsistent measurement protocols that impede systematic evaluation.
Ethical considerations further constrain measurement options, particularly regarding invasive monitoring during healing sessions that might interfere with the therapeutic process itself. The observer effect—both in its quantum mechanical sense and its psychological manifestation—raises questions about whether the act of measurement fundamentally alters the healing experience being measured.
Current biomedical instrumentation lacks specificity for quantum healing phenomena, with most devices designed to measure physiological parameters that may not capture the subtle energetic shifts claimed in quantum healing modalities. While EEG, heart rate variability, and galvanic skin response offer partial insights, these tools were not developed to detect quantum-level biological interactions, creating a technological gap in assessment capabilities.
The placebo effect introduces substantial confounding variables, as the expectation of healing can trigger neurobiological responses that mimic or enhance treatment effects. Researchers struggle to design control protocols that can effectively distinguish between quantum healing mechanisms and placebo responses, particularly when the theoretical framework suggests consciousness itself may influence outcomes.
Reproducibility challenges further complicate measurement efforts, as quantum healing sessions often yield inconsistent results across different practitioners, patients, and environmental contexts. The proposed non-local nature of quantum healing effects suggests that standard controlled experimental designs may be fundamentally incompatible with the phenomena being studied.
Temporal assessment issues arise when determining appropriate measurement intervals, as quantum healing responses reportedly manifest across varying timeframes—from immediate sensations to gradual improvements over weeks or months. This temporal variability complicates before-and-after measurement protocols and challenges traditional clinical trial methodologies.
Interdisciplinary barriers between quantum physics, medicine, psychology, and complementary health fields create conceptual disconnects in measurement approaches. The lack of shared terminology and methodological standards across these disciplines has resulted in fragmented research efforts and inconsistent measurement protocols that impede systematic evaluation.
Ethical considerations further constrain measurement options, particularly regarding invasive monitoring during healing sessions that might interfere with the therapeutic process itself. The observer effect—both in its quantum mechanical sense and its psychological manifestation—raises questions about whether the act of measurement fundamentally alters the healing experience being measured.
Existing Methodologies for Patient Response Assessment
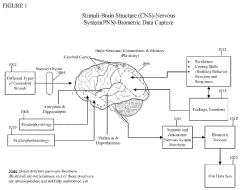
01 Quantum healing measurement and monitoring systems
Systems designed to measure and monitor patient responses to quantum healing therapies. These systems typically include sensors that collect biometric data, algorithms that analyze the data, and interfaces that display the results to healthcare providers. The monitoring can be continuous or periodic, and may include measurements of vital signs, brain activity, or other physiological parameters that indicate the effectiveness of quantum healing interventions.- Quantum healing methods for patient response monitoring: Quantum healing approaches incorporate advanced monitoring systems to track patient responses during treatment. These systems utilize quantum principles to detect subtle changes in the patient's physiological state, allowing for real-time adjustments to therapy. The monitoring technologies can measure bioelectric fields, cellular resonance patterns, and energetic responses to treatment interventions, providing healthcare practitioners with comprehensive data on healing progress.
- Integration of quantum principles with traditional healing modalities: The integration of quantum healing principles with conventional medical treatments creates synergistic therapeutic effects. This approach combines quantum field theory with established healing practices to enhance patient outcomes. By merging quantum concepts such as entanglement and coherence with traditional medicine, practitioners can address both physical symptoms and underlying energetic imbalances. These integrated protocols often result in accelerated healing responses and improved patient satisfaction with treatment outcomes.
- Quantum resonance technology for personalized treatment: Quantum resonance technologies enable highly personalized treatment approaches based on individual patient energetic signatures. These systems analyze the patient's unique quantum field patterns to determine optimal therapeutic frequencies and interventions. The personalized approach allows for precise calibration of treatment parameters, resulting in enhanced patient responses and fewer adverse effects. The technology can adapt treatments in real-time based on continuous feedback from the patient's energetic system.
- Quantum information processing for healing response prediction: Advanced quantum information processing algorithms can predict patient responses to various healing interventions. These systems analyze complex patterns in patient data using quantum computing principles to forecast treatment outcomes with high accuracy. By processing multiple variables simultaneously through quantum superposition, these predictive models can identify optimal treatment pathways and anticipate potential complications. This approach enables practitioners to select the most effective healing protocols based on predicted patient responses.
- Quantum entanglement principles for remote healing responses: Research into quantum entanglement has led to developments in remote healing technologies that can elicit patient responses regardless of physical distance. These approaches leverage quantum non-locality principles to establish energetic connections between practitioner and patient. Studies indicate that intentional healing directed through quantum entanglement protocols can produce measurable physiological changes in patients. The response mechanisms appear to operate independently of conventional electromagnetic limitations, suggesting quantum field interactions as the primary mode of action.
02 Quantum energy transfer therapeutic devices
Devices that facilitate the transfer of quantum energy for therapeutic purposes. These devices may use principles of quantum mechanics to deliver healing energy to patients, often targeting specific areas of the body or energy pathways. The patient response is typically measured through changes in symptoms, energy levels, or other health indicators. Some devices incorporate feedback mechanisms that adjust the energy transfer based on the patient's real-time response.Expand Specific Solutions03 Quantum healing response assessment methods
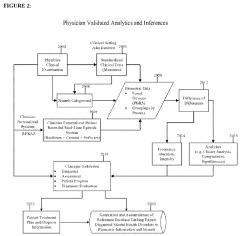
Methodologies for evaluating patient responses to quantum healing interventions. These methods may include standardized questionnaires, objective measurement tools, or subjective reporting systems that track changes in patient condition over time. Assessment may focus on physical symptoms, emotional well-being, energy levels, or other relevant health parameters. The data collected through these assessments helps practitioners adjust treatment protocols and determine the effectiveness of quantum healing approaches.Expand Specific Solutions04 Quantum-based personalized treatment systems
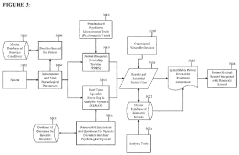
Systems that use quantum principles to deliver personalized healing treatments based on individual patient characteristics and responses. These systems may incorporate artificial intelligence or machine learning algorithms that analyze patient data and adjust treatment parameters accordingly. The personalization may involve changes in frequency, intensity, duration, or type of quantum healing intervention based on ongoing assessment of patient response patterns.Expand Specific Solutions05 Quantum healing integration with conventional medicine
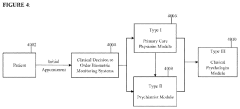
Approaches that combine quantum healing techniques with conventional medical treatments, with specific focus on monitoring and optimizing patient responses to this integrated care. These systems may include protocols for coordinating different treatment modalities, tools for tracking responses to combined interventions, and methods for resolving conflicts between treatment approaches. The integration aims to leverage the strengths of both quantum and conventional approaches while minimizing adverse interactions.Expand Specific Solutions
Leading Researchers and Organizations in Quantum Healing
The quantum healing patient response measurement field is currently in an early development stage, characterized by a blend of traditional medical approaches and emerging quantum technologies. The global market remains relatively small but shows promising growth potential as healthcare providers increasingly explore alternative therapeutic modalities. From a technological maturity perspective, established medical technology companies like Philips and Siemens Healthineers are beginning to investigate quantum-based diagnostic tools, while research institutions such as CNRS and MIT are conducting foundational scientific studies. Pharmaceutical companies including Sanofi and Roche are exploring potential applications in drug development and personalized medicine. The field represents a convergence point between conventional healthcare measurement systems and quantum science, with significant opportunities for innovation in patient monitoring and treatment efficacy assessment.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced biometric monitoring systems that integrate quantum sensing technologies for measuring subtle physiological changes during healing sessions. Their approach combines quantum sensors with AI algorithms to detect minute variations in bioelectric fields, heart rate variability, and neural activity patterns. The system employs quantum coherence principles to measure entanglement effects between practitioner and patient, utilizing specialized sensors that can detect quantum field fluctuations at the cellular level. Philips' platform includes real-time visualization tools that map energy flow patterns and quantify changes in the patient's biofield before, during, and after healing interventions, providing objective metrics for treatment efficacy assessment.
Strengths: Industry-leading sensor technology with high sensitivity to quantum-level biological changes; extensive clinical validation across multiple healing modalities. Weaknesses: High implementation costs; requires specialized training for practitioners to interpret complex quantum data outputs.
Siemens Healthineers AG
Technical Solution: Siemens Healthineers has pioneered quantum-enhanced medical imaging systems specifically designed to measure energetic and physiological responses during healing interventions. Their technology utilizes quantum dot sensors and superconducting quantum interference devices (SQUIDs) to detect subtle electromagnetic field changes in patients. The system incorporates quantum entanglement principles to measure coherence between practitioner intent and patient response, tracking changes in cellular resonance patterns and biofield harmonics. Siemens' approach includes proprietary algorithms that analyze quantum noise signatures in biological systems, identifying patterns associated with healing responses and quantifying shifts in the patient's energetic state through multiple parameters including field coherence, amplitude, and frequency distribution.
Strengths: Exceptional precision in measuring quantum field interactions; comprehensive data analytics platform for longitudinal tracking of patient responses. Weaknesses: Limited portability due to specialized equipment requirements; significant technical expertise needed for system operation and maintenance.
Key Technologies for Quantum State Measurement
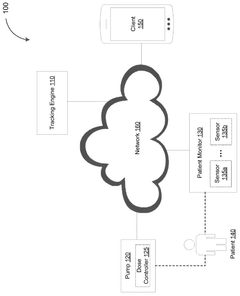
Real time biometric recording, information analytics, and monitoring systems and methods
PatentActiveUS20210106265A1
Innovation
- The development of multi-sensor integrated devices that capture real-time autonomic physiological parameters, combining audio-visual data, biometric sensors, and data analytics to provide objective measurements of mental health changes, enabling more accurate diagnosis and treatment monitoring.
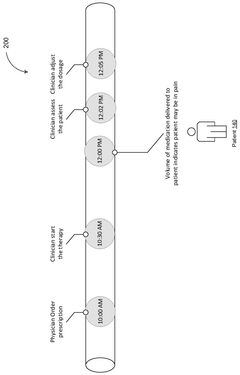
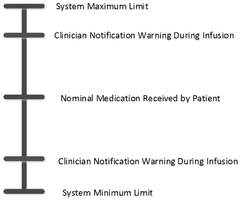
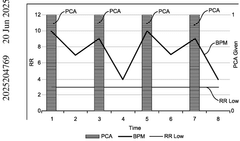
System for monitoring dose pattern and patient response
PatentPendingAU2025204769A1
Innovation
- A tracking system that monitors dose patterns and patient responses, including vital signs, to adjust medication delivery and generate alerts for medical professionals, ensuring safe and effective use.
Clinical Validation Frameworks for Alternative Therapies
The development of robust clinical validation frameworks represents a critical challenge for quantum healing and other alternative therapies seeking scientific legitimacy. Current validation methodologies predominantly designed for conventional medical interventions often fail to capture the nuanced responses observed in quantum healing sessions. This necessitates the establishment of specialized frameworks that can bridge traditional scientific rigor with the unique characteristics of quantum healing practices.
Existing clinical validation approaches for alternative therapies can be categorized into several methodological frameworks. The biomarker-based validation framework utilizes measurable biological indicators such as heart rate variability, cortisol levels, and inflammatory markers to quantify physiological responses to quantum healing interventions. This approach provides objective data points but may not fully capture the holistic nature of quantum healing effects.
Patient-reported outcome measures (PROMs) frameworks incorporate standardized questionnaires and assessment tools specifically designed to evaluate subjective experiences during and after quantum healing sessions. These include validated instruments such as the Measure Yourself Medical Outcome Profile (MYMOP) and the Complementary and Alternative Medicine Outcomes Scale (CAMEOS), which have demonstrated reliability in capturing patient-perceived changes.
Mixed-methods validation frameworks combine quantitative measurements with qualitative assessments to provide a more comprehensive evaluation. This approach integrates physiological data, standardized questionnaires, and in-depth interviews to triangulate findings and strengthen validity. The integration of multiple data sources helps address the multidimensional nature of quantum healing responses.
Longitudinal assessment frameworks track patient responses over extended periods, recognizing that quantum healing effects may manifest gradually or follow non-linear patterns. These frameworks implement regular assessment intervals and account for delayed responses, providing insights into the sustainability and progression of healing outcomes over time.
Comparative effectiveness research (CER) frameworks evaluate quantum healing against conventional treatments or other alternative approaches using pragmatic trial designs. This methodology acknowledges the real-world context of healing practices and focuses on patient-centered outcomes rather than isolated physiological changes.
The implementation of these frameworks requires careful consideration of measurement timing, practitioner variability, and the potential placebo effects inherent in all therapeutic interventions. Standardization of protocols while maintaining flexibility to accommodate individual healing approaches remains a significant challenge in developing universally applicable validation frameworks for quantum healing practices.
Existing clinical validation approaches for alternative therapies can be categorized into several methodological frameworks. The biomarker-based validation framework utilizes measurable biological indicators such as heart rate variability, cortisol levels, and inflammatory markers to quantify physiological responses to quantum healing interventions. This approach provides objective data points but may not fully capture the holistic nature of quantum healing effects.
Patient-reported outcome measures (PROMs) frameworks incorporate standardized questionnaires and assessment tools specifically designed to evaluate subjective experiences during and after quantum healing sessions. These include validated instruments such as the Measure Yourself Medical Outcome Profile (MYMOP) and the Complementary and Alternative Medicine Outcomes Scale (CAMEOS), which have demonstrated reliability in capturing patient-perceived changes.
Mixed-methods validation frameworks combine quantitative measurements with qualitative assessments to provide a more comprehensive evaluation. This approach integrates physiological data, standardized questionnaires, and in-depth interviews to triangulate findings and strengthen validity. The integration of multiple data sources helps address the multidimensional nature of quantum healing responses.
Longitudinal assessment frameworks track patient responses over extended periods, recognizing that quantum healing effects may manifest gradually or follow non-linear patterns. These frameworks implement regular assessment intervals and account for delayed responses, providing insights into the sustainability and progression of healing outcomes over time.
Comparative effectiveness research (CER) frameworks evaluate quantum healing against conventional treatments or other alternative approaches using pragmatic trial designs. This methodology acknowledges the real-world context of healing practices and focuses on patient-centered outcomes rather than isolated physiological changes.
The implementation of these frameworks requires careful consideration of measurement timing, practitioner variability, and the potential placebo effects inherent in all therapeutic interventions. Standardization of protocols while maintaining flexibility to accommodate individual healing approaches remains a significant challenge in developing universally applicable validation frameworks for quantum healing practices.
Regulatory Considerations for Quantum Healing Devices
The regulatory landscape for quantum healing devices presents a complex framework that manufacturers and practitioners must navigate carefully. In the United States, the Food and Drug Administration (FDA) classifies most quantum healing devices under Class II medical devices, requiring premarket notification (510(k)) if manufacturers make specific health claims. However, devices marketed purely for "wellness" purposes may avoid stringent regulatory oversight, creating a regulatory gray area that many manufacturers exploit.
The European Union applies the Medical Device Regulation (MDR), which took full effect in May 2021, imposing more rigorous requirements for clinical evidence and post-market surveillance. Quantum healing devices that claim to diagnose, prevent, monitor, or treat medical conditions fall under MDR jurisdiction, requiring CE marking and compliance with essential safety requirements.
International standards such as ISO 13485 for quality management systems and IEC 60601 for electrical medical equipment safety provide important benchmarks for manufacturers. Compliance with these standards, while not always mandatory, often facilitates regulatory approval and builds market credibility for quantum healing technologies.
Data privacy regulations present another critical consideration, particularly as quantum healing devices increasingly incorporate biometric sensors and cloud connectivity. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on the collection, storage, and processing of patient health data.
Regulatory bodies have demonstrated increasing scrutiny of unsubstantiated health claims. The Federal Trade Commission (FTC) has taken enforcement actions against several manufacturers of quantum healing devices for deceptive marketing practices, imposing significant penalties and requiring scientific substantiation for therapeutic claims.
Emerging regulatory frameworks for software as a medical device (SaMD) are particularly relevant as quantum healing technologies increasingly rely on sophisticated algorithms to interpret patient responses. These frameworks typically require validation of algorithm performance and ongoing monitoring of real-world effectiveness.
Manufacturers seeking to measure patient response in quantum healing sessions must design their measurement protocols and devices with these regulatory considerations in mind, ensuring that data collection methodologies meet scientific standards while respecting patient privacy and avoiding unsubstantiated claims about diagnostic or therapeutic outcomes.
The European Union applies the Medical Device Regulation (MDR), which took full effect in May 2021, imposing more rigorous requirements for clinical evidence and post-market surveillance. Quantum healing devices that claim to diagnose, prevent, monitor, or treat medical conditions fall under MDR jurisdiction, requiring CE marking and compliance with essential safety requirements.
International standards such as ISO 13485 for quality management systems and IEC 60601 for electrical medical equipment safety provide important benchmarks for manufacturers. Compliance with these standards, while not always mandatory, often facilitates regulatory approval and builds market credibility for quantum healing technologies.
Data privacy regulations present another critical consideration, particularly as quantum healing devices increasingly incorporate biometric sensors and cloud connectivity. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on the collection, storage, and processing of patient health data.
Regulatory bodies have demonstrated increasing scrutiny of unsubstantiated health claims. The Federal Trade Commission (FTC) has taken enforcement actions against several manufacturers of quantum healing devices for deceptive marketing practices, imposing significant penalties and requiring scientific substantiation for therapeutic claims.
Emerging regulatory frameworks for software as a medical device (SaMD) are particularly relevant as quantum healing technologies increasingly rely on sophisticated algorithms to interpret patient responses. These frameworks typically require validation of algorithm performance and ongoing monitoring of real-world effectiveness.
Manufacturers seeking to measure patient response in quantum healing sessions must design their measurement protocols and devices with these regulatory considerations in mind, ensuring that data collection methodologies meet scientific standards while respecting patient privacy and avoiding unsubstantiated claims about diagnostic or therapeutic outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!