Kevlar Applications in Breakthrough Wound Treatment Devices
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kevlar in Wound Care: Background and Objectives
Kevlar, a high-strength synthetic fiber developed by DuPont in the 1960s, has long been associated with protective applications such as bulletproof vests and aerospace components. However, its unique properties have recently caught the attention of medical researchers and innovators in the field of wound treatment. This technological shift marks a significant evolution in the application of Kevlar, expanding its potential beyond traditional industrial and military uses.
The journey of Kevlar in wound care began with the recognition of its exceptional strength-to-weight ratio, biocompatibility, and resistance to degradation. These characteristics make it an ideal candidate for advanced wound treatment devices, particularly in scenarios where conventional materials fall short. The primary objective of incorporating Kevlar into wound care is to develop more effective, durable, and versatile treatment solutions that can address complex wound healing challenges.
As the global wound care market continues to grow, driven by factors such as an aging population and the rising incidence of chronic wounds, the need for innovative treatment approaches becomes increasingly apparent. Kevlar-based wound treatment devices aim to address several key challenges in current wound care practices, including the need for improved tensile strength in wound closure materials, enhanced protection against external contaminants, and the development of smart, responsive wound dressings.
The technological evolution of Kevlar in wound care is closely aligned with broader trends in medical materials science, such as the development of nanofiber technologies and smart materials that can respond to changes in the wound environment. Researchers are exploring ways to leverage Kevlar's unique molecular structure to create advanced wound healing platforms that can accelerate tissue regeneration, reduce scarring, and minimize the risk of infection.
One of the most promising aspects of Kevlar in wound treatment is its potential to be combined with other advanced materials and technologies. For instance, the integration of Kevlar fibers with bioactive compounds or drug delivery systems could lead to multifunctional wound dressings that not only provide mechanical support but also actively promote healing and fight infection. This synergistic approach represents a significant leap forward in wound care technology, potentially revolutionizing treatment protocols for both acute and chronic wounds.
As research in this field progresses, the ultimate goal is to develop a new generation of wound treatment devices that offer superior performance, improved patient outcomes, and reduced healthcare costs. The application of Kevlar in this context exemplifies the broader trend of cross-disciplinary innovation, where materials originally designed for one purpose find groundbreaking applications in entirely different fields, driving technological advancement and improving quality of life.
The journey of Kevlar in wound care began with the recognition of its exceptional strength-to-weight ratio, biocompatibility, and resistance to degradation. These characteristics make it an ideal candidate for advanced wound treatment devices, particularly in scenarios where conventional materials fall short. The primary objective of incorporating Kevlar into wound care is to develop more effective, durable, and versatile treatment solutions that can address complex wound healing challenges.
As the global wound care market continues to grow, driven by factors such as an aging population and the rising incidence of chronic wounds, the need for innovative treatment approaches becomes increasingly apparent. Kevlar-based wound treatment devices aim to address several key challenges in current wound care practices, including the need for improved tensile strength in wound closure materials, enhanced protection against external contaminants, and the development of smart, responsive wound dressings.
The technological evolution of Kevlar in wound care is closely aligned with broader trends in medical materials science, such as the development of nanofiber technologies and smart materials that can respond to changes in the wound environment. Researchers are exploring ways to leverage Kevlar's unique molecular structure to create advanced wound healing platforms that can accelerate tissue regeneration, reduce scarring, and minimize the risk of infection.
One of the most promising aspects of Kevlar in wound treatment is its potential to be combined with other advanced materials and technologies. For instance, the integration of Kevlar fibers with bioactive compounds or drug delivery systems could lead to multifunctional wound dressings that not only provide mechanical support but also actively promote healing and fight infection. This synergistic approach represents a significant leap forward in wound care technology, potentially revolutionizing treatment protocols for both acute and chronic wounds.
As research in this field progresses, the ultimate goal is to develop a new generation of wound treatment devices that offer superior performance, improved patient outcomes, and reduced healthcare costs. The application of Kevlar in this context exemplifies the broader trend of cross-disciplinary innovation, where materials originally designed for one purpose find groundbreaking applications in entirely different fields, driving technological advancement and improving quality of life.
Market Analysis for Advanced Wound Treatment Solutions
The advanced wound treatment solutions market has been experiencing significant growth in recent years, driven by an increasing prevalence of chronic wounds, a growing aging population, and rising incidences of diabetes and obesity. This market segment encompasses a wide range of products and technologies designed to promote faster healing, reduce infection risks, and improve patient outcomes.
The global advanced wound care market was valued at approximately $10.3 billion in 2020 and is projected to reach $15.7 billion by 2026, growing at a CAGR of 7.2% during the forecast period. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high healthcare expenditure.
Key factors driving market growth include the rising incidence of chronic wounds, such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers. The International Diabetes Federation estimates that the number of adults with diabetes will increase from 463 million in 2019 to 700 million by 2045, potentially leading to a surge in diabetic wound cases.
The market is segmented into various product categories, including advanced dressings, wound therapy devices, and active wound care products. Advanced dressings, which include foam dressings, hydrocolloids, and alginates, currently hold the largest market share. However, wound therapy devices, such as negative pressure wound therapy (NPWT) systems, are expected to witness the highest growth rate in the coming years.
Technological advancements play a crucial role in shaping the market landscape. Innovations in materials science, including the potential application of Kevlar in wound treatment devices, are opening new avenues for product development. The integration of smart technologies, such as sensors for wound monitoring and AI-powered treatment optimization, is also gaining traction.
The competitive landscape of the advanced wound treatment solutions market is characterized by the presence of both established players and innovative start-ups. Key market players include 3M Company, Smith & Nephew, Mölnlycke Health Care, and ConvaTec Group. These companies are investing heavily in research and development to maintain their competitive edge and expand their product portfolios.
Emerging trends in the market include a shift towards personalized wound care solutions, increased focus on bioactive and biomaterial-based products, and the development of combination therapies that address multiple aspects of wound healing simultaneously. The potential integration of Kevlar into wound treatment devices represents an exciting area of innovation that could significantly impact the market in the coming years.
The global advanced wound care market was valued at approximately $10.3 billion in 2020 and is projected to reach $15.7 billion by 2026, growing at a CAGR of 7.2% during the forecast period. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to its advanced healthcare infrastructure and high healthcare expenditure.
Key factors driving market growth include the rising incidence of chronic wounds, such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers. The International Diabetes Federation estimates that the number of adults with diabetes will increase from 463 million in 2019 to 700 million by 2045, potentially leading to a surge in diabetic wound cases.
The market is segmented into various product categories, including advanced dressings, wound therapy devices, and active wound care products. Advanced dressings, which include foam dressings, hydrocolloids, and alginates, currently hold the largest market share. However, wound therapy devices, such as negative pressure wound therapy (NPWT) systems, are expected to witness the highest growth rate in the coming years.
Technological advancements play a crucial role in shaping the market landscape. Innovations in materials science, including the potential application of Kevlar in wound treatment devices, are opening new avenues for product development. The integration of smart technologies, such as sensors for wound monitoring and AI-powered treatment optimization, is also gaining traction.
The competitive landscape of the advanced wound treatment solutions market is characterized by the presence of both established players and innovative start-ups. Key market players include 3M Company, Smith & Nephew, Mölnlycke Health Care, and ConvaTec Group. These companies are investing heavily in research and development to maintain their competitive edge and expand their product portfolios.
Emerging trends in the market include a shift towards personalized wound care solutions, increased focus on bioactive and biomaterial-based products, and the development of combination therapies that address multiple aspects of wound healing simultaneously. The potential integration of Kevlar into wound treatment devices represents an exciting area of innovation that could significantly impact the market in the coming years.
Current Challenges in Kevlar-Based Wound Care Devices
Despite the remarkable properties of Kevlar in wound treatment devices, several challenges persist in its application and integration into advanced medical technologies. One of the primary obstacles is the difficulty in achieving optimal biocompatibility. While Kevlar exhibits excellent mechanical strength and durability, its interaction with living tissues can sometimes lead to inflammatory responses or delayed healing processes. This necessitates the development of surface modification techniques or composite materials that can enhance the biological performance of Kevlar-based devices without compromising their mechanical advantages.
Another significant challenge lies in the manufacturing processes required to incorporate Kevlar into complex wound care devices. The high melting point and limited solubility of Kevlar make it challenging to process using conventional methods. This often results in increased production costs and limitations in design flexibility, particularly when creating intricate structures or integrating Kevlar with other materials essential for wound healing, such as hydrogels or drug-delivery systems.
The long-term performance and degradation of Kevlar in physiological environments also present ongoing concerns. While Kevlar is known for its durability, its behavior under prolonged exposure to bodily fluids and enzymes is not fully understood. This uncertainty raises questions about the long-term efficacy and safety of Kevlar-based wound care devices, especially in chronic wound management scenarios where extended use is required.
Furthermore, the development of Kevlar-based smart wound dressings faces technical hurdles in seamlessly integrating sensing and responsive elements. The challenge lies in maintaining the structural integrity and protective properties of Kevlar while incorporating features such as real-time monitoring of wound healing progress or controlled release of therapeutic agents. This integration often requires innovative approaches in material science and nanotechnology.
Regulatory compliance and standardization pose additional challenges. The unique properties of Kevlar and its relatively novel application in wound care devices necessitate rigorous testing and validation processes. Establishing standardized protocols for assessing the safety and efficacy of Kevlar-based medical devices is crucial but complex, given the material's distinct characteristics compared to traditional wound care materials.
Lastly, the cost-effectiveness of Kevlar-based wound treatment devices remains a significant barrier to widespread adoption. While the material offers superior performance in many aspects, the high production costs and specialized manufacturing requirements can make these devices prohibitively expensive for routine use in healthcare settings. Balancing the advanced capabilities of Kevlar-based devices with economic viability is an ongoing challenge that requires innovative approaches in both material science and production technologies.
Another significant challenge lies in the manufacturing processes required to incorporate Kevlar into complex wound care devices. The high melting point and limited solubility of Kevlar make it challenging to process using conventional methods. This often results in increased production costs and limitations in design flexibility, particularly when creating intricate structures or integrating Kevlar with other materials essential for wound healing, such as hydrogels or drug-delivery systems.
The long-term performance and degradation of Kevlar in physiological environments also present ongoing concerns. While Kevlar is known for its durability, its behavior under prolonged exposure to bodily fluids and enzymes is not fully understood. This uncertainty raises questions about the long-term efficacy and safety of Kevlar-based wound care devices, especially in chronic wound management scenarios where extended use is required.
Furthermore, the development of Kevlar-based smart wound dressings faces technical hurdles in seamlessly integrating sensing and responsive elements. The challenge lies in maintaining the structural integrity and protective properties of Kevlar while incorporating features such as real-time monitoring of wound healing progress or controlled release of therapeutic agents. This integration often requires innovative approaches in material science and nanotechnology.
Regulatory compliance and standardization pose additional challenges. The unique properties of Kevlar and its relatively novel application in wound care devices necessitate rigorous testing and validation processes. Establishing standardized protocols for assessing the safety and efficacy of Kevlar-based medical devices is crucial but complex, given the material's distinct characteristics compared to traditional wound care materials.
Lastly, the cost-effectiveness of Kevlar-based wound treatment devices remains a significant barrier to widespread adoption. While the material offers superior performance in many aspects, the high production costs and specialized manufacturing requirements can make these devices prohibitively expensive for routine use in healthcare settings. Balancing the advanced capabilities of Kevlar-based devices with economic viability is an ongoing challenge that requires innovative approaches in both material science and production technologies.
Existing Kevlar-Integrated Wound Treatment Solutions
01 Kevlar-reinforced composite materials
Kevlar fibers are used to reinforce various composite materials, enhancing their strength, durability, and impact resistance. These composites find applications in aerospace, automotive, and protective equipment industries. The incorporation of Kevlar improves the overall performance and lightweight properties of the resulting materials.- Kevlar-reinforced composite materials: Kevlar fibers are incorporated into various composite materials to enhance their strength, durability, and impact resistance. These composites find applications in aerospace, automotive, and protective equipment industries. The combination of Kevlar with other materials like carbon fiber or polymers creates lightweight yet robust structures.
- Kevlar-based protective gear: Kevlar is extensively used in the development of personal protective equipment, including bulletproof vests, helmets, and cut-resistant gloves. Its high tensile strength and heat-resistant properties make it ideal for creating lightweight yet effective protective gear for military, law enforcement, and industrial applications.
- Kevlar in textile and clothing applications: Kevlar fibers are integrated into various textile products to improve their durability, tear resistance, and heat protection. This includes applications in high-performance sportswear, fire-resistant clothing, and industrial workwear. The incorporation of Kevlar enhances the overall performance and longevity of these textile products.
- Kevlar-enhanced automotive components: Kevlar is utilized in the automotive industry to manufacture lightweight yet strong components. It is incorporated into tires, belts, hoses, and body panels to improve fuel efficiency, durability, and safety. The use of Kevlar in automotive applications contributes to overall vehicle performance and longevity.
- Kevlar in advanced manufacturing processes: Innovative manufacturing techniques are developed to process and integrate Kevlar into various products. This includes advanced weaving methods, 3D printing with Kevlar-reinforced materials, and novel coating processes. These manufacturing advancements enable the creation of complex Kevlar-based structures with tailored properties for specific applications.
02 Kevlar-based protective gear and clothing
Kevlar is utilized in the production of protective gear and clothing, such as bulletproof vests, helmets, and cut-resistant gloves. Its high tensile strength and heat-resistant properties make it ideal for personal protection equipment in military, law enforcement, and industrial applications.Expand Specific Solutions03 Kevlar applications in automotive industry
The automotive industry employs Kevlar in various components to improve vehicle performance and safety. It is used in tire reinforcement, brake pads, and body panels to reduce weight while maintaining strength. Kevlar-reinforced parts contribute to improved fuel efficiency and crash resistance.Expand Specific Solutions04 Kevlar in aerospace and marine applications
Kevlar finds extensive use in aerospace and marine industries due to its lightweight and high-strength properties. It is employed in aircraft and spacecraft components, as well as in boat hulls and sails. The material's resistance to fatigue and corrosion makes it suitable for these demanding environments.Expand Specific Solutions05 Kevlar-based fire-resistant materials
Kevlar is utilized in the development of fire-resistant materials and clothing. Its inherent flame-retardant properties make it suitable for firefighting gear, industrial protective clothing, and building materials. These applications help improve safety in high-risk environments and reduce the spread of fires.Expand Specific Solutions
Key Players in Kevlar-Enhanced Medical Devices
The Kevlar applications in breakthrough wound treatment devices market is in an early growth stage, characterized by increasing research and development efforts. The market size is expanding as innovative companies like Shockwave Medical, Smith & Nephew, and Surmodics explore Kevlar's potential in medical applications. While the technology is still emerging, its maturity is advancing rapidly due to collaborations between industry leaders and research institutions such as Huazhong University of Science & Technology and Drexel University. Companies like 3M Innovative Properties and BSN medical are leveraging their expertise in materials science to develop novel Kevlar-based wound treatment solutions, indicating a competitive landscape with diverse players from medical device, materials, and healthcare sectors.
Smith & Nephew plc
Technical Solution: Smith & Nephew has integrated Kevlar technology into their advanced wound care portfolio, focusing on creating robust yet flexible wound dressings for challenging wound environments. Their approach involves blending Kevlar fibers with biocompatible polymers to create a composite material that offers exceptional strength and conformability. This composite is used in multi-layered dressings designed for high-exudate wounds, where the Kevlar-enhanced layer provides structural integrity and helps maintain the dressing's shape even when saturated [4]. The company has also developed a line of Kevlar-reinforced negative pressure wound therapy (NPWT) dressings, which can withstand higher pressures and offer improved durability for complex wounds requiring long-term NPWT application [5].
Strengths: High durability in challenging wound environments, improved efficacy for complex wounds. Weaknesses: May be more expensive than traditional dressings, potential for limited flexibility in some applications.
KCI Licensing, Inc.
Technical Solution: KCI Licensing, a subsidiary of 3M, has pioneered the use of Kevlar in advanced wound healing devices, particularly in their negative pressure wound therapy (NPWT) systems. Their innovative approach involves incorporating Kevlar fibers into the foam dressings used in NPWT, creating a more resilient and durable interface between the wound and the negative pressure source. This Kevlar-enhanced foam can withstand higher pressures and maintain its structural integrity even in highly exudating wounds, allowing for more effective fluid removal and granulation tissue formation [6]. Additionally, KCI has developed a Kevlar-reinforced drape for their NPWT systems, which provides a stronger seal around the wound and reduces the risk of air leaks, thereby improving the overall efficacy of the therapy [7].
Strengths: Enhanced durability and efficacy in NPWT applications, improved wound sealing capabilities. Weaknesses: Potentially higher cost compared to standard NPWT components, may require specialized training for healthcare providers.
Innovative Kevlar Technologies for Wound Healing
Coating composition
PatentActiveUS20120021038A1
Innovation
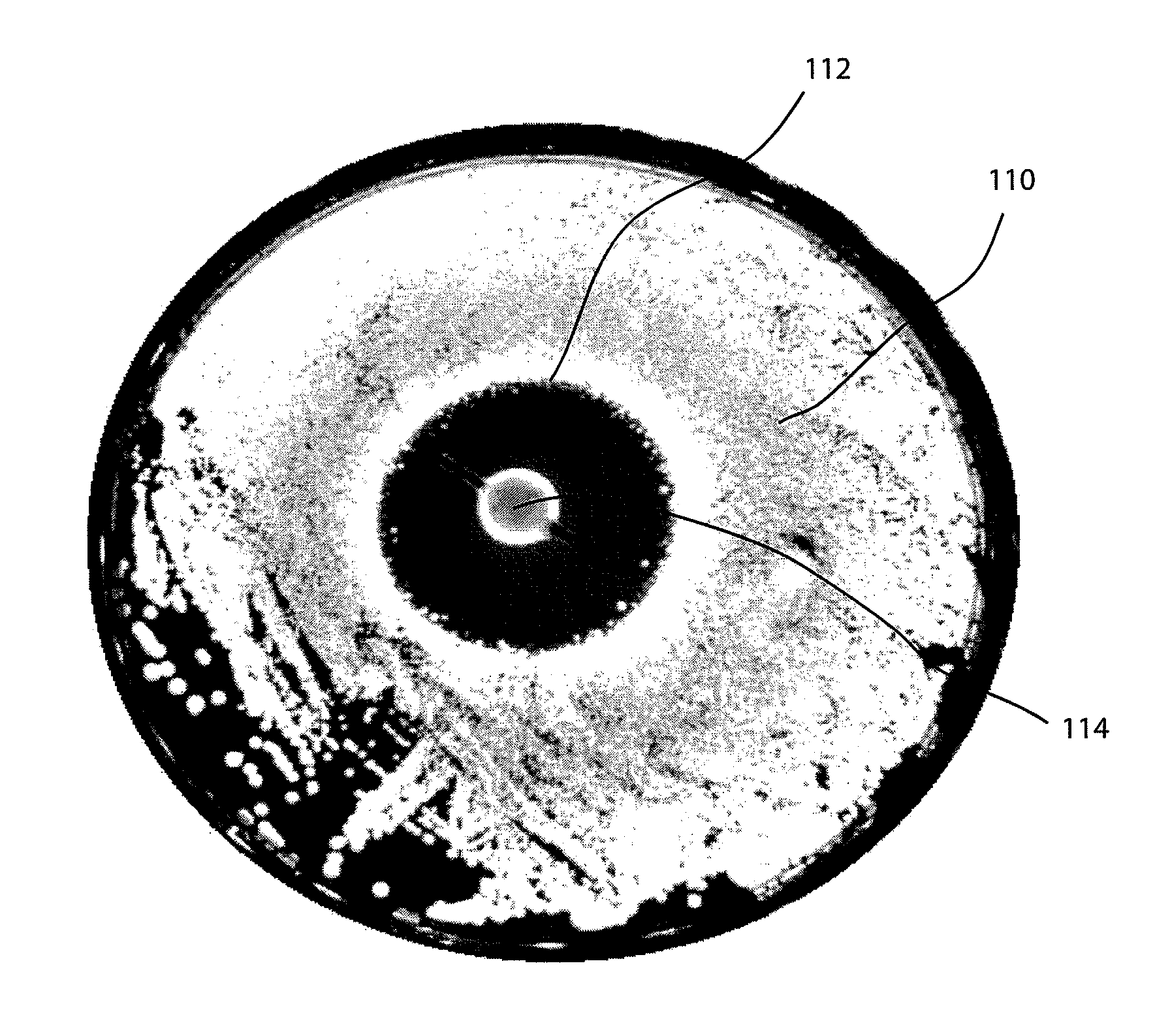
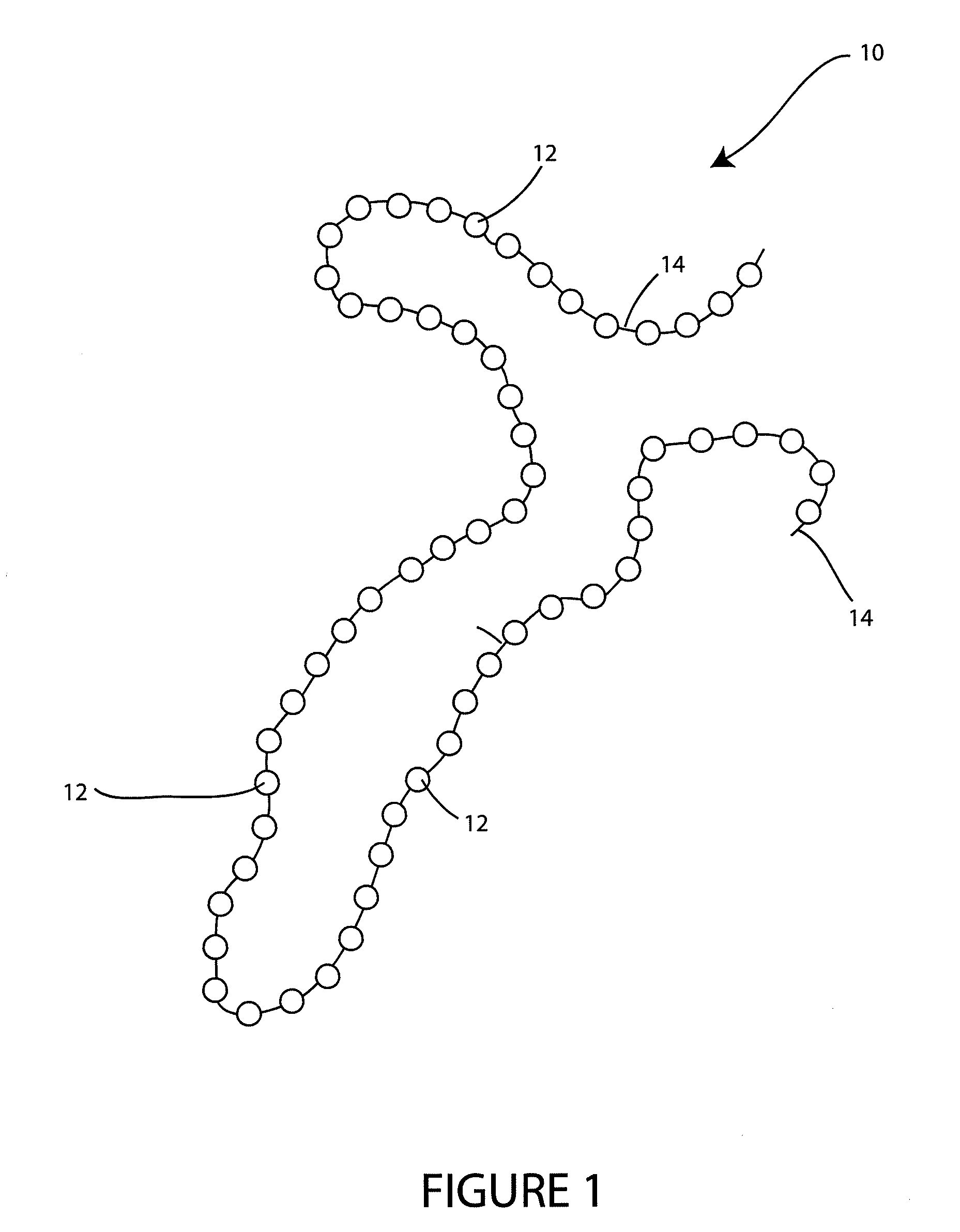
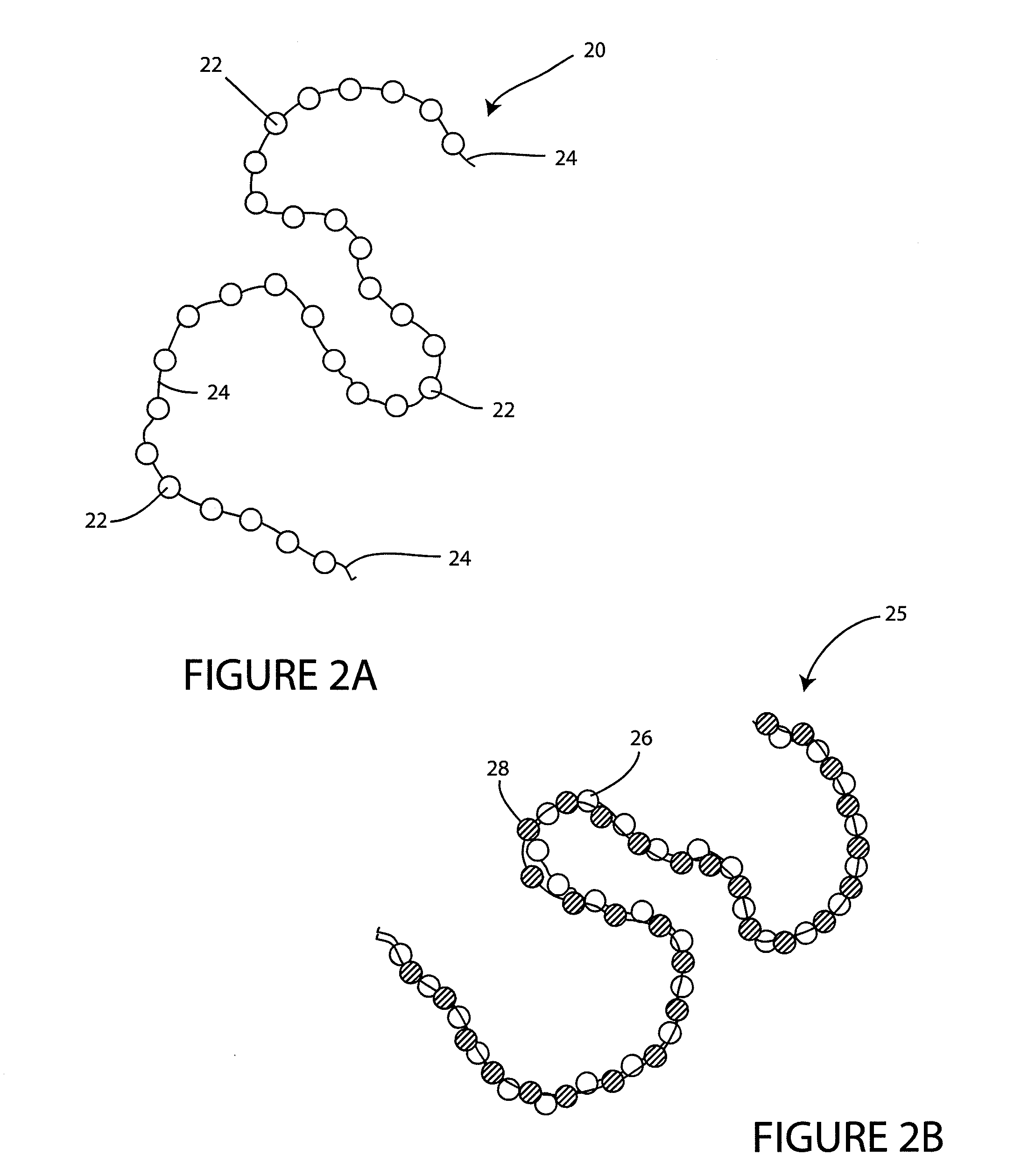
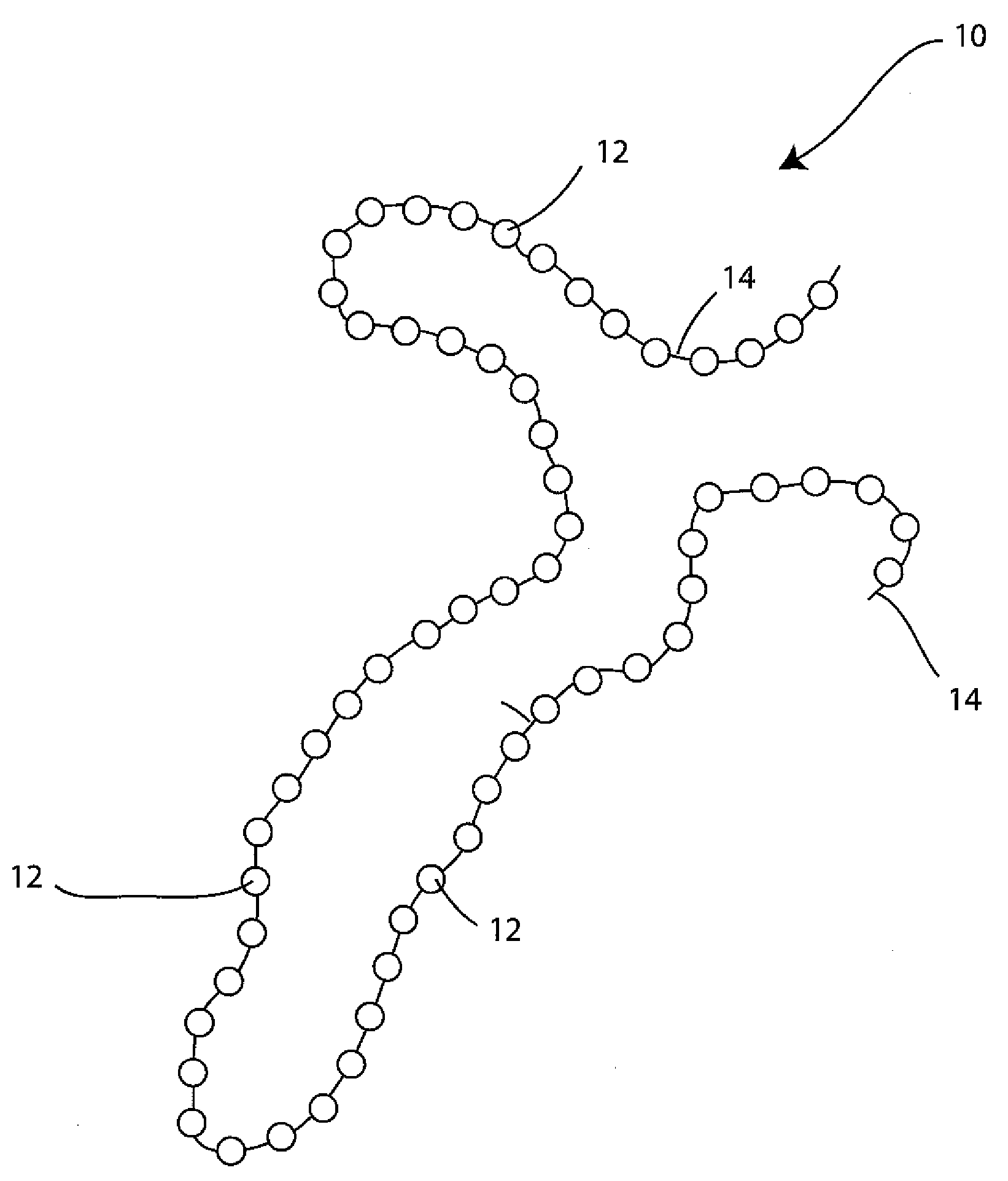
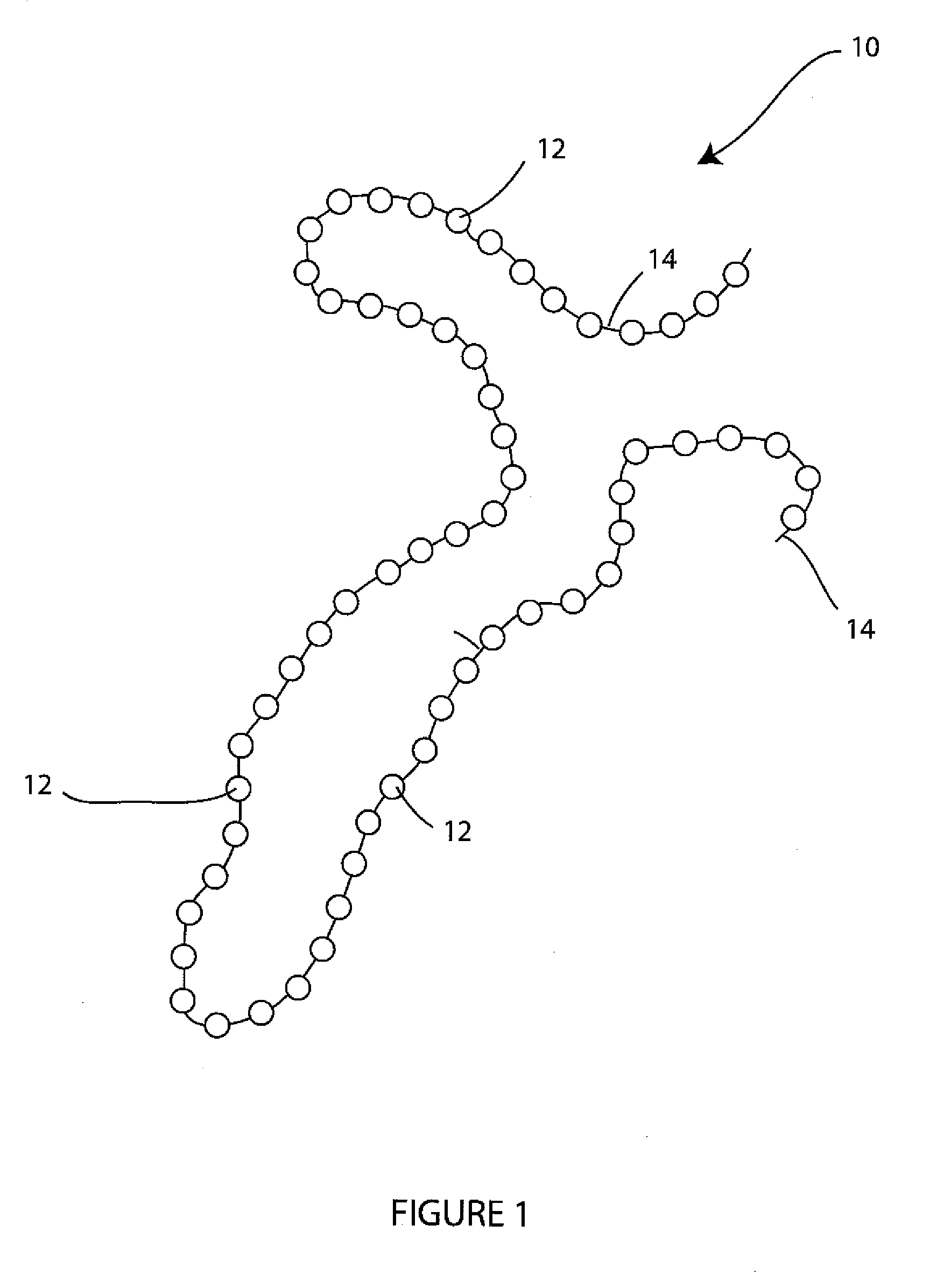
- A wound spacer device comprising multiple beads connected by non-absorbable suture material, designed for controlled elution of antimicrobial agents like Tobramycin, allowing for site-specific and controlled release over a defined period, while maintaining the wound in an open condition to prevent infection and tissue damage.
Medical device with coating composition
PatentInactiveUS20090202609A1
Innovation
- A coating composition applied under controlled humidity conditions to implantable medical devices, allowing for controlled release of bioactive agents, such as antibiotics, which accelerates release under increased humidity and decelerates release under decreased humidity, and is designed for use in devices that undergo flexion or expansion, including a wound spacer device with antimicrobial beads for wound management.
Regulatory Framework for Kevlar in Medical Devices
The regulatory framework for Kevlar in medical devices is a complex and evolving landscape that requires careful navigation by manufacturers and healthcare providers. The U.S. Food and Drug Administration (FDA) plays a pivotal role in overseeing the use of Kevlar in wound treatment devices, classifying them based on their intended use and potential risks.
Under the FDA's regulatory structure, Kevlar-based wound treatment devices typically fall into Class II, which requires special controls in addition to general controls. This classification necessitates manufacturers to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
The European Union's regulatory approach, governed by the Medical Device Regulation (MDR), imposes stringent requirements for Kevlar-based medical devices. Manufacturers must obtain CE marking, which involves a comprehensive conformity assessment process, including clinical evaluation and risk management.
In both the U.S. and EU markets, manufacturers must adhere to Good Manufacturing Practices (GMP) and implement quality management systems to ensure consistent production of safe and effective devices. This includes rigorous testing of Kevlar materials for biocompatibility, durability, and performance under various conditions.
Regulatory bodies also focus on the potential long-term effects of Kevlar in wound treatment applications. Manufacturers are required to conduct post-market surveillance and report any adverse events or complications associated with their devices. This ongoing monitoring helps regulatory agencies refine their guidelines and ensure patient safety.
The use of Kevlar in medical devices also intersects with regulations governing advanced materials and nanotechnology. As Kevlar fibers can be engineered at the nanoscale, additional considerations may apply regarding potential environmental and health impacts.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline regulatory processes across different countries. This initiative allows manufacturers to undergo a single audit to satisfy the regulatory requirements of multiple jurisdictions, potentially expediting the global market access for innovative Kevlar-based wound treatment devices.
As the field of wound treatment continues to advance, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay abreast of these changes and engage proactively with regulatory bodies to ensure compliance and facilitate the introduction of novel Kevlar applications in medical devices.
Under the FDA's regulatory structure, Kevlar-based wound treatment devices typically fall into Class II, which requires special controls in addition to general controls. This classification necessitates manufacturers to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
The European Union's regulatory approach, governed by the Medical Device Regulation (MDR), imposes stringent requirements for Kevlar-based medical devices. Manufacturers must obtain CE marking, which involves a comprehensive conformity assessment process, including clinical evaluation and risk management.
In both the U.S. and EU markets, manufacturers must adhere to Good Manufacturing Practices (GMP) and implement quality management systems to ensure consistent production of safe and effective devices. This includes rigorous testing of Kevlar materials for biocompatibility, durability, and performance under various conditions.
Regulatory bodies also focus on the potential long-term effects of Kevlar in wound treatment applications. Manufacturers are required to conduct post-market surveillance and report any adverse events or complications associated with their devices. This ongoing monitoring helps regulatory agencies refine their guidelines and ensure patient safety.
The use of Kevlar in medical devices also intersects with regulations governing advanced materials and nanotechnology. As Kevlar fibers can be engineered at the nanoscale, additional considerations may apply regarding potential environmental and health impacts.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline regulatory processes across different countries. This initiative allows manufacturers to undergo a single audit to satisfy the regulatory requirements of multiple jurisdictions, potentially expediting the global market access for innovative Kevlar-based wound treatment devices.
As the field of wound treatment continues to advance, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay abreast of these changes and engage proactively with regulatory bodies to ensure compliance and facilitate the introduction of novel Kevlar applications in medical devices.
Biocompatibility and Safety Considerations
The biocompatibility and safety considerations of Kevlar applications in breakthrough wound treatment devices are paramount to ensure patient well-being and regulatory compliance. Kevlar, primarily known for its use in protective gear, presents unique challenges when incorporated into medical devices that come into direct contact with human tissue.
One of the primary concerns is the potential for adverse reactions at the wound site. While Kevlar itself is generally considered inert, its application in wound treatment devices necessitates rigorous testing to evaluate any possible inflammatory responses or allergic reactions. Long-term studies are essential to assess the material's impact on wound healing processes and to rule out any chronic effects that may not be immediately apparent.
The surface properties of Kevlar fibers play a crucial role in their biocompatibility. Modifications to the fiber surface may be necessary to enhance cell adhesion and promote tissue integration. Researchers are exploring various coating techniques and surface treatments to optimize the interaction between Kevlar-based devices and the surrounding biological environment.
Another critical aspect is the potential for particle shedding. As Kevlar fibers may degrade over time, especially in the dynamic environment of a healing wound, it is vital to evaluate the risk of micro or nanoparticles being released into the tissue. These particles could potentially migrate to other parts of the body, raising concerns about systemic toxicity and long-term health effects.
The sterilization process for Kevlar-based wound treatment devices presents additional challenges. Traditional sterilization methods may affect the material properties of Kevlar, potentially compromising its performance or safety. Developing and validating sterilization protocols that maintain the integrity of the Kevlar components while ensuring complete microbial elimination is a key area of focus for researchers and manufacturers.
Regulatory bodies, such as the FDA and EMA, require extensive safety data before approving new materials for use in medical devices. This includes comprehensive biocompatibility testing following ISO 10993 standards, which cover aspects such as cytotoxicity, sensitization, and genotoxicity. Manufacturers must also conduct thorough risk assessments to identify and mitigate potential hazards associated with the use of Kevlar in wound treatment applications.
As research progresses, there is a growing interest in developing biodegradable variants of Kevlar or Kevlar-like materials that can be safely absorbed by the body over time. This approach could potentially address some of the long-term safety concerns associated with permanent implantation of synthetic materials in wound treatment devices.
One of the primary concerns is the potential for adverse reactions at the wound site. While Kevlar itself is generally considered inert, its application in wound treatment devices necessitates rigorous testing to evaluate any possible inflammatory responses or allergic reactions. Long-term studies are essential to assess the material's impact on wound healing processes and to rule out any chronic effects that may not be immediately apparent.
The surface properties of Kevlar fibers play a crucial role in their biocompatibility. Modifications to the fiber surface may be necessary to enhance cell adhesion and promote tissue integration. Researchers are exploring various coating techniques and surface treatments to optimize the interaction between Kevlar-based devices and the surrounding biological environment.
Another critical aspect is the potential for particle shedding. As Kevlar fibers may degrade over time, especially in the dynamic environment of a healing wound, it is vital to evaluate the risk of micro or nanoparticles being released into the tissue. These particles could potentially migrate to other parts of the body, raising concerns about systemic toxicity and long-term health effects.
The sterilization process for Kevlar-based wound treatment devices presents additional challenges. Traditional sterilization methods may affect the material properties of Kevlar, potentially compromising its performance or safety. Developing and validating sterilization protocols that maintain the integrity of the Kevlar components while ensuring complete microbial elimination is a key area of focus for researchers and manufacturers.
Regulatory bodies, such as the FDA and EMA, require extensive safety data before approving new materials for use in medical devices. This includes comprehensive biocompatibility testing following ISO 10993 standards, which cover aspects such as cytotoxicity, sensitization, and genotoxicity. Manufacturers must also conduct thorough risk assessments to identify and mitigate potential hazards associated with the use of Kevlar in wound treatment applications.
As research progresses, there is a growing interest in developing biodegradable variants of Kevlar or Kevlar-like materials that can be safely absorbed by the body over time. This approach could potentially address some of the long-term safety concerns associated with permanent implantation of synthetic materials in wound treatment devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!