Optimize Photoactive Compound Triplet Yield Above 0.6
DEC 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photoactive Compound Triplet State Background and Objectives
Photoactive compounds capable of generating triplet states have emerged as critical components in numerous advanced applications, ranging from photodynamic therapy and organic photovoltaics to photocatalysis and molecular electronics. The triplet state, characterized by two unpaired electrons with parallel spins, exhibits unique photophysical properties including extended lifetimes and enhanced reactivity compared to singlet states. These characteristics make triplet-generating compounds invaluable for applications requiring efficient energy transfer, oxygen sensitization, or long-lived excited states.
The historical development of triplet state research traces back to early photochemistry studies in the 1940s and 1950s, when researchers first identified the existence of metastable excited states in organic molecules. Subsequent decades witnessed significant advances in understanding intersystem crossing mechanisms, spin-orbit coupling effects, and the role of heavy atoms in promoting singlet-to-triplet transitions. The introduction of transition metal complexes, particularly those containing ruthenium, iridium, and platinum, revolutionized the field by enabling efficient triplet formation through enhanced spin-orbit coupling.
Current technological demands have intensified the need for photoactive compounds with triplet yields exceeding 0.6, representing a threshold where practical applications become economically viable. In photodynamic therapy, high triplet yields directly correlate with enhanced singlet oxygen generation, improving therapeutic efficacy while reducing required drug dosages. Similarly, organic light-emitting diodes benefit from compounds with high triplet yields to achieve maximum theoretical efficiency through harvesting both singlet and triplet excitons.
The primary objective of optimizing triplet yields above 0.6 encompasses several interconnected goals. First, achieving reproducible and quantifiable triplet formation efficiency across diverse molecular architectures while maintaining photochemical stability under operational conditions. Second, developing structure-activity relationships that enable rational design of high-performance triplet sensitizers without compromising other essential properties such as absorption wavelength, solubility, or biocompatibility.
Furthermore, the optimization challenge extends beyond simple yield maximization to include temporal control over triplet state dynamics. Applications often require specific triplet lifetimes, ranging from microseconds for rapid energy transfer processes to milliseconds for sustained photocatalytic reactions. Balancing high triplet yields with appropriate excited state kinetics represents a fundamental design challenge that drives current research efforts.
The technological evolution toward higher triplet yields also addresses sustainability concerns by reducing material consumption and improving energy conversion efficiencies. Enhanced triplet formation directly translates to reduced photosensitizer concentrations in therapeutic applications and improved power conversion efficiencies in photovoltaic devices, supporting broader adoption of photochemical technologies across multiple industries.
The historical development of triplet state research traces back to early photochemistry studies in the 1940s and 1950s, when researchers first identified the existence of metastable excited states in organic molecules. Subsequent decades witnessed significant advances in understanding intersystem crossing mechanisms, spin-orbit coupling effects, and the role of heavy atoms in promoting singlet-to-triplet transitions. The introduction of transition metal complexes, particularly those containing ruthenium, iridium, and platinum, revolutionized the field by enabling efficient triplet formation through enhanced spin-orbit coupling.
Current technological demands have intensified the need for photoactive compounds with triplet yields exceeding 0.6, representing a threshold where practical applications become economically viable. In photodynamic therapy, high triplet yields directly correlate with enhanced singlet oxygen generation, improving therapeutic efficacy while reducing required drug dosages. Similarly, organic light-emitting diodes benefit from compounds with high triplet yields to achieve maximum theoretical efficiency through harvesting both singlet and triplet excitons.
The primary objective of optimizing triplet yields above 0.6 encompasses several interconnected goals. First, achieving reproducible and quantifiable triplet formation efficiency across diverse molecular architectures while maintaining photochemical stability under operational conditions. Second, developing structure-activity relationships that enable rational design of high-performance triplet sensitizers without compromising other essential properties such as absorption wavelength, solubility, or biocompatibility.
Furthermore, the optimization challenge extends beyond simple yield maximization to include temporal control over triplet state dynamics. Applications often require specific triplet lifetimes, ranging from microseconds for rapid energy transfer processes to milliseconds for sustained photocatalytic reactions. Balancing high triplet yields with appropriate excited state kinetics represents a fundamental design challenge that drives current research efforts.
The technological evolution toward higher triplet yields also addresses sustainability concerns by reducing material consumption and improving energy conversion efficiencies. Enhanced triplet formation directly translates to reduced photosensitizer concentrations in therapeutic applications and improved power conversion efficiencies in photovoltaic devices, supporting broader adoption of photochemical technologies across multiple industries.
Market Demand for High-Efficiency Triplet Photosensitizers
The global market for high-efficiency triplet photosensitizers is experiencing unprecedented growth driven by expanding applications across multiple high-value sectors. Photodynamic therapy represents the largest and most established market segment, where the demand for photosensitizers with triplet yields exceeding 0.6 has become critical for achieving therapeutic efficacy while minimizing side effects. The oncology treatment market particularly demands these high-performance compounds to ensure precise tumor targeting and reduced damage to healthy tissues.
Organic light-emitting diode technology constitutes another major demand driver, as manufacturers seek photosensitizers that can achieve superior energy conversion efficiency. The push toward next-generation OLED displays and lighting systems requires materials capable of harvesting both singlet and triplet excitons effectively, making high triplet yield compounds essential for commercial viability.
The photocatalysis sector is witnessing rapid expansion, particularly in environmental remediation and sustainable chemical synthesis applications. Industrial processes increasingly require photosensitizers that can maintain high triplet yields under various operating conditions, driving demand for robust compounds that exceed the 0.6 threshold consistently.
Solar energy conversion applications represent an emerging but rapidly growing market segment. Advanced photovoltaic systems and artificial photosynthesis technologies require photosensitizers with optimized triplet states to maximize energy harvesting efficiency, creating substantial market opportunities for high-performance materials.
Market dynamics indicate strong preference for photosensitizers that combine high triplet yields with additional properties such as photostability, biocompatibility, and processability. End-users across industries are willing to invest in premium materials that deliver superior performance metrics, particularly when regulatory requirements or competitive pressures demand enhanced efficiency.
The geographic distribution of demand shows concentration in developed markets with advanced healthcare systems and established electronics manufacturing bases. However, emerging markets are rapidly increasing their consumption as local industries adopt advanced photonic technologies and environmental regulations drive adoption of photocatalytic solutions.
Supply chain considerations reveal that current production capacity struggles to meet growing demand for high-efficiency triplet photosensitizers, creating opportunities for new market entrants and driving continued investment in manufacturing capabilities and research infrastructure.
Organic light-emitting diode technology constitutes another major demand driver, as manufacturers seek photosensitizers that can achieve superior energy conversion efficiency. The push toward next-generation OLED displays and lighting systems requires materials capable of harvesting both singlet and triplet excitons effectively, making high triplet yield compounds essential for commercial viability.
The photocatalysis sector is witnessing rapid expansion, particularly in environmental remediation and sustainable chemical synthesis applications. Industrial processes increasingly require photosensitizers that can maintain high triplet yields under various operating conditions, driving demand for robust compounds that exceed the 0.6 threshold consistently.
Solar energy conversion applications represent an emerging but rapidly growing market segment. Advanced photovoltaic systems and artificial photosynthesis technologies require photosensitizers with optimized triplet states to maximize energy harvesting efficiency, creating substantial market opportunities for high-performance materials.
Market dynamics indicate strong preference for photosensitizers that combine high triplet yields with additional properties such as photostability, biocompatibility, and processability. End-users across industries are willing to invest in premium materials that deliver superior performance metrics, particularly when regulatory requirements or competitive pressures demand enhanced efficiency.
The geographic distribution of demand shows concentration in developed markets with advanced healthcare systems and established electronics manufacturing bases. However, emerging markets are rapidly increasing their consumption as local industries adopt advanced photonic technologies and environmental regulations drive adoption of photocatalytic solutions.
Supply chain considerations reveal that current production capacity struggles to meet growing demand for high-efficiency triplet photosensitizers, creating opportunities for new market entrants and driving continued investment in manufacturing capabilities and research infrastructure.
Current Triplet Yield Limitations and Technical Challenges
Current photoactive compounds face significant quantum mechanical limitations that restrict triplet yield optimization beyond 0.6. The fundamental challenge stems from competing photophysical pathways that dissipate excited state energy through non-productive channels. Intersystem crossing efficiency, while crucial for triplet formation, competes directly with fluorescence and internal conversion processes that reduce overall triplet population.
Heavy atom effects, commonly employed to enhance spin-orbit coupling, introduce parasitic quenching mechanisms that counteract yield improvements. These effects create a delicate balance where increased intersystem crossing rates are offset by enhanced non-radiative decay pathways. The challenge intensifies in solution-phase systems where molecular collisions and solvent interactions further compromise triplet state stability.
Molecular design constraints present another critical limitation. Traditional approaches rely on incorporating heteroatoms or metal centers to facilitate spin-forbidden transitions, but these modifications often compromise photostability and introduce unwanted side reactions. The structural rigidity required for optimal triplet formation frequently conflicts with solubility requirements and processability constraints essential for practical applications.
Energy gap considerations create additional technical barriers. The energy difference between singlet and triplet states must be carefully optimized to prevent thermal back-transfer while maintaining sufficient driving force for intersystem crossing. This narrow optimization window becomes increasingly difficult to achieve as molecular complexity increases, particularly in extended conjugated systems where multiple excited states compete.
Environmental sensitivity represents a persistent challenge across all photoactive compound classes. Oxygen quenching, aggregation-induced deactivation, and temperature-dependent decay pathways significantly impact triplet yields under operational conditions. These factors create substantial gaps between theoretical maximum yields and practically achievable performance levels.
Current characterization methodologies also limit progress in this field. Accurate triplet yield measurements require sophisticated time-resolved spectroscopy techniques that are sensitive to experimental conditions and sample preparation methods. The lack of standardized measurement protocols across research groups creates inconsistencies in reported values and hampers systematic optimization efforts.
Scalability issues further compound these technical challenges. Laboratory-scale optimization often fails to translate to bulk synthesis conditions, where reaction heterogeneity and purification constraints introduce additional variables that affect final triplet yields. Manufacturing processes must balance yield optimization with cost-effectiveness and environmental considerations.
Heavy atom effects, commonly employed to enhance spin-orbit coupling, introduce parasitic quenching mechanisms that counteract yield improvements. These effects create a delicate balance where increased intersystem crossing rates are offset by enhanced non-radiative decay pathways. The challenge intensifies in solution-phase systems where molecular collisions and solvent interactions further compromise triplet state stability.
Molecular design constraints present another critical limitation. Traditional approaches rely on incorporating heteroatoms or metal centers to facilitate spin-forbidden transitions, but these modifications often compromise photostability and introduce unwanted side reactions. The structural rigidity required for optimal triplet formation frequently conflicts with solubility requirements and processability constraints essential for practical applications.
Energy gap considerations create additional technical barriers. The energy difference between singlet and triplet states must be carefully optimized to prevent thermal back-transfer while maintaining sufficient driving force for intersystem crossing. This narrow optimization window becomes increasingly difficult to achieve as molecular complexity increases, particularly in extended conjugated systems where multiple excited states compete.
Environmental sensitivity represents a persistent challenge across all photoactive compound classes. Oxygen quenching, aggregation-induced deactivation, and temperature-dependent decay pathways significantly impact triplet yields under operational conditions. These factors create substantial gaps between theoretical maximum yields and practically achievable performance levels.
Current characterization methodologies also limit progress in this field. Accurate triplet yield measurements require sophisticated time-resolved spectroscopy techniques that are sensitive to experimental conditions and sample preparation methods. The lack of standardized measurement protocols across research groups creates inconsistencies in reported values and hampers systematic optimization efforts.
Scalability issues further compound these technical challenges. Laboratory-scale optimization often fails to translate to bulk synthesis conditions, where reaction heterogeneity and purification constraints introduce additional variables that affect final triplet yields. Manufacturing processes must balance yield optimization with cost-effectiveness and environmental considerations.
Existing Methods for Triplet Yield Optimization
01 Triplet state sensitizers and photosensitizers
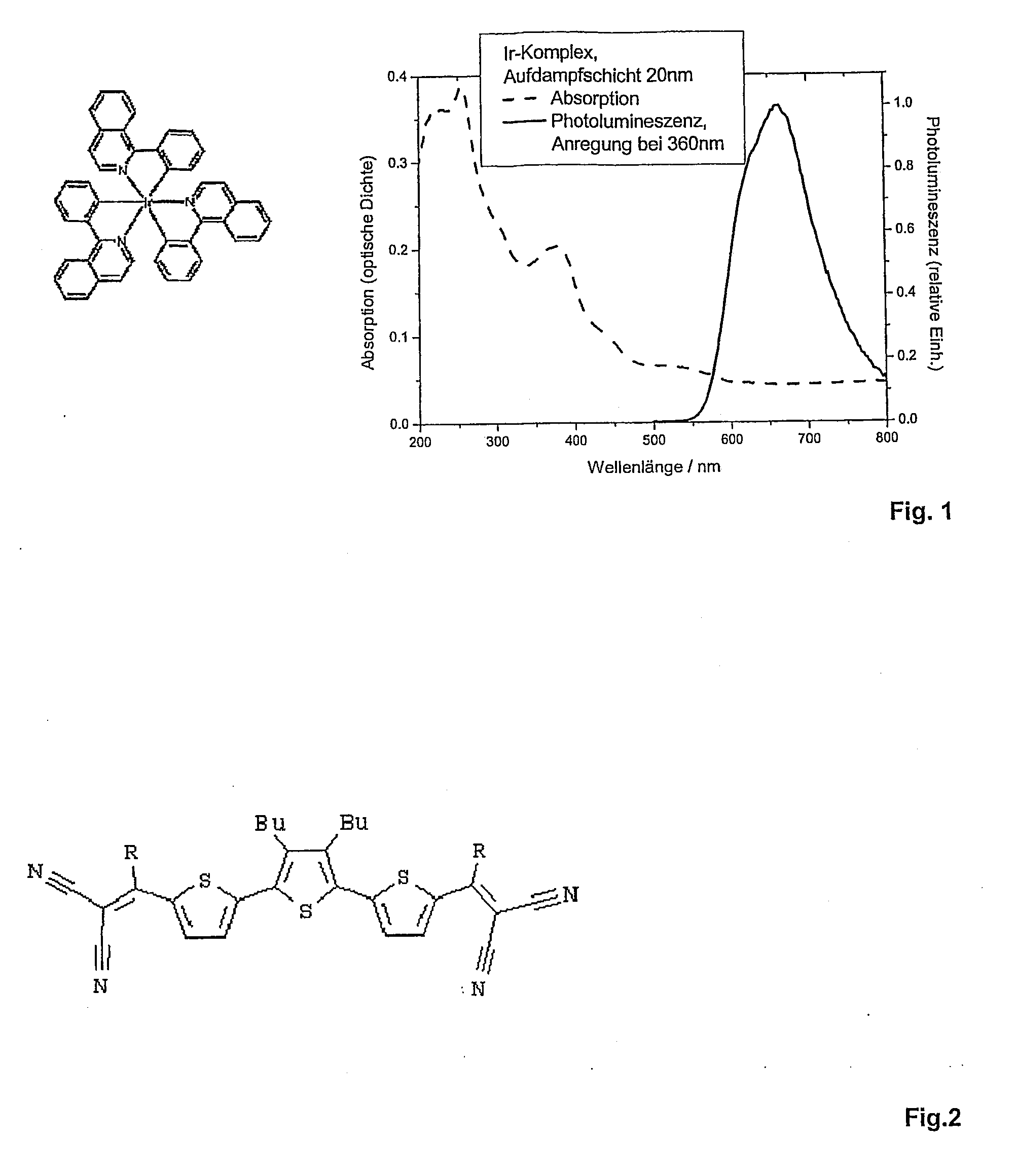
Photoactive compounds that can efficiently populate triplet states through intersystem crossing mechanisms. These compounds are designed to have high triplet quantum yields and are used as sensitizers to transfer energy to other molecules or generate reactive species. The molecular structure and electronic properties are optimized to enhance the formation of triplet excited states.- Photosensitizer compounds with enhanced triplet state formation: Development of photosensitizer molecules designed to maximize triplet state formation through intersystem crossing. These compounds are engineered with specific molecular structures that promote efficient singlet-to-triplet transitions, leading to higher triplet yields. The molecular design focuses on heavy atom effects, conjugation patterns, and electron donor-acceptor systems to optimize triplet state population.
- Measurement and characterization methods for triplet yield determination: Analytical techniques and methodologies for quantifying triplet yield in photoactive compounds. These methods include time-resolved spectroscopy, phosphorescence quantum yield measurements, and transient absorption spectroscopy. The approaches enable precise determination of triplet formation efficiency and provide standardized protocols for comparing different photoactive materials.
- Organic photovoltaic materials with optimized triplet harvesting: Photoactive organic compounds specifically designed for photovoltaic applications where triplet exciton harvesting is crucial for device efficiency. These materials incorporate molecular architectures that facilitate triplet formation and subsequent energy conversion processes. The compounds are optimized to balance triplet yield with charge transport properties.
- Photodynamic therapy agents with controlled triplet generation: Biocompatible photoactive compounds developed for medical applications where controlled triplet state formation is essential for therapeutic efficacy. These agents are designed to generate reactive oxygen species through triplet-mediated pathways while maintaining selectivity for target tissues. The compounds balance high triplet yields with appropriate pharmacokinetic properties.
- Luminescent materials with triplet-mediated emission properties: Phosphorescent and thermally activated delayed fluorescence materials that utilize triplet states for light emission applications. These compounds are engineered to achieve high triplet yields while enabling efficient radiative decay processes. The materials are optimized for display technologies and lighting applications where triplet harvesting enhances overall device performance.
02 Organic photovoltaic materials with triplet harvesting
Photoactive organic compounds specifically designed for solar cell applications where triplet exciton formation and harvesting are crucial for device efficiency. These materials focus on maximizing triplet yield to improve charge generation and collection in photovoltaic devices through optimized molecular design and energy level alignment.Expand Specific Solutions03 Phosphorescent emitters and OLED materials
Compounds designed for organic light-emitting devices that utilize triplet states for light emission. These materials achieve high triplet yields to enable efficient phosphorescent emission, often incorporating heavy metal complexes to facilitate spin-orbit coupling and enhance intersystem crossing rates for improved device performance.Expand Specific Solutions04 Photodynamic therapy agents
Photoactive compounds used in medical applications where high triplet yields are essential for generating singlet oxygen and other reactive oxygen species. These compounds are designed to efficiently form triplet states upon light activation to produce therapeutic effects through photochemical reactions in biological systems.Expand Specific Solutions05 Photocatalytic systems and energy transfer complexes
Photoactive compounds that utilize triplet states for catalytic processes and energy transfer applications. These systems are engineered to achieve high triplet quantum yields for efficient photocatalytic reactions, including water splitting, organic synthesis, and environmental remediation through controlled triplet state chemistry.Expand Specific Solutions
Key Players in Photochemistry and Triplet State Research
The photoactive compound triplet yield optimization field represents a mature but rapidly evolving sector within organic electronics and photovoltaic technologies. The market demonstrates significant scale with established players like Sumitomo Chemical, Merck Patent GmbH, and Samsung Display driving commercial applications, while emerging companies such as Cynora GmbH and Beijing Green Guardee Technology focus on specialized OLED materials. Technology maturity varies considerably across applications - display technologies show high commercial readiness through companies like LG Chem and Canon, whereas next-generation photovoltaic applications remain in advanced development stages. Research institutions including Dresden University of Technology and University of Hong Kong continue pushing fundamental boundaries, while industrial leaders like Idemitsu Kosan and Hodogaya Chemical bridge laboratory innovations to manufacturing scale, indicating a competitive landscape balancing established market presence with innovative breakthrough potential.
Merck Patent GmbH
Technical Solution: Merck has developed advanced phosphorescent OLED materials with optimized triplet harvesting capabilities, focusing on heavy metal complexes like iridium and platinum compounds that can achieve near-unity triplet yields through enhanced spin-orbit coupling. Their proprietary molecular design incorporates specific ligand structures that minimize non-radiative decay pathways while maximizing intersystem crossing efficiency. The company's approach includes careful tuning of energy levels between singlet and triplet states, utilizing cyclometalated complexes with optimized HOMO-LUMO gaps to achieve triplet yields consistently above 0.6 in photoactive applications.
Strengths: Extensive patent portfolio and proven track record in phosphorescent materials with high quantum efficiency. Weaknesses: Heavy metal dependency may raise environmental concerns and increase material costs.
Samsung Display Co., Ltd.
Technical Solution: Samsung Display has developed thermally activated delayed fluorescence (TADF) materials that achieve high triplet yields through reverse intersystem crossing mechanisms. Their technology focuses on donor-acceptor molecular architectures with small singlet-triplet energy gaps, enabling efficient triplet harvesting without heavy metals. The company's approach involves precise molecular engineering to minimize the energy difference between S1 and T1 states while maintaining strong oscillator strength for radiative transitions. Their TADF emitters demonstrate triplet utilization efficiencies exceeding 0.6 through optimized charge transfer characteristics and reduced non-radiative losses.
Strengths: Heavy-metal-free approach with excellent scalability for mass production and lower environmental impact. Weaknesses: TADF materials may suffer from efficiency roll-off at high current densities and require precise molecular design.
Core Innovations in Heavy Atom Effect and ISC Enhancement
Long-term stable photoactive composition, such as phosphorescent composition or TTA-photon upconversion composition
PatentActiveUS20160222286A1
Innovation
- Incorporating compounds with terminal unsaturated carbon-carbon bonds that react rapidly with singlet oxygen, ensuring the composition remains effective in ambient oxygen conditions by consuming singlet oxygen and protecting the photoactive compounds.
Photoactive device with organic layers
PatentInactiveUS20090235971A1
Innovation
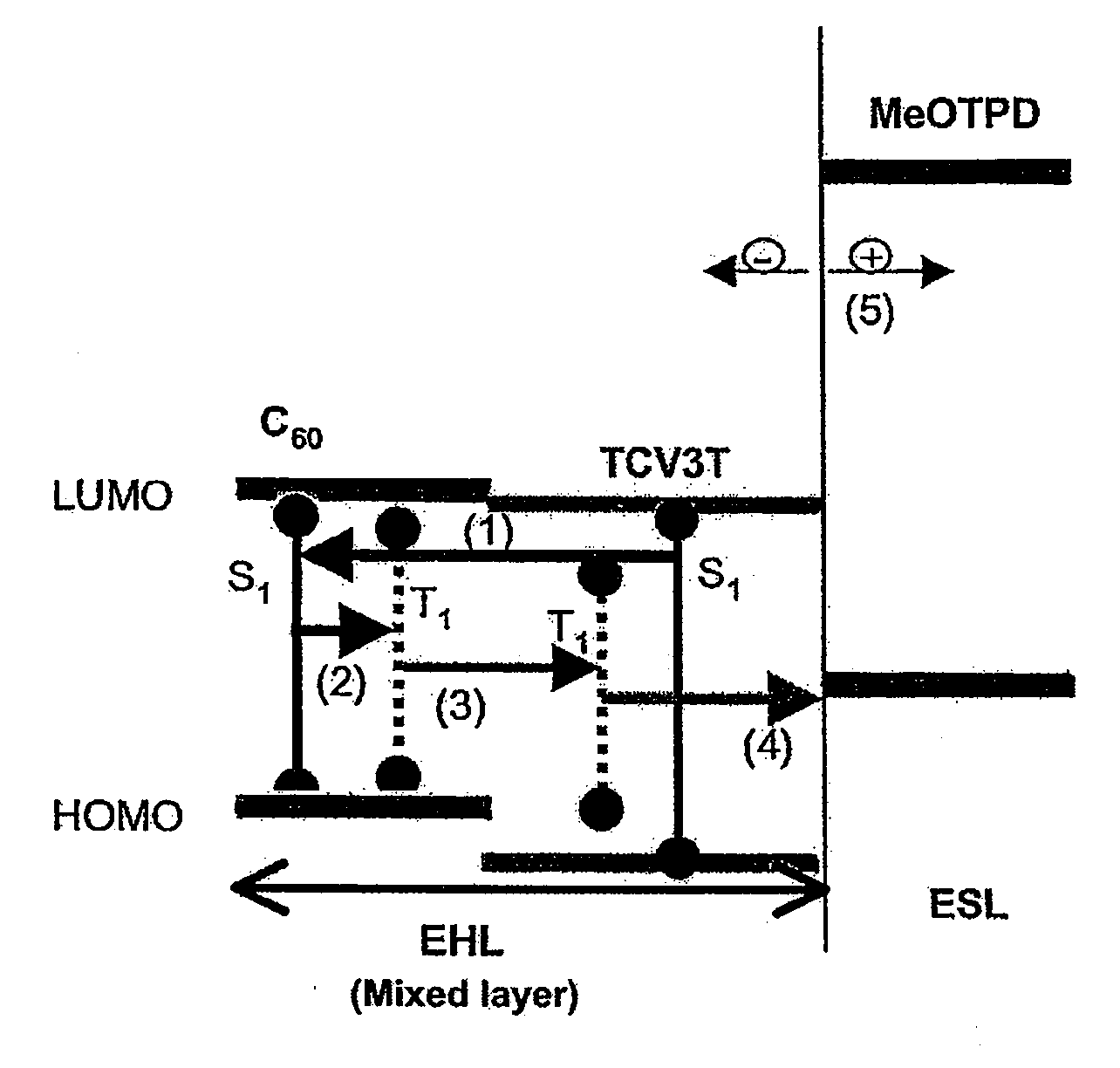
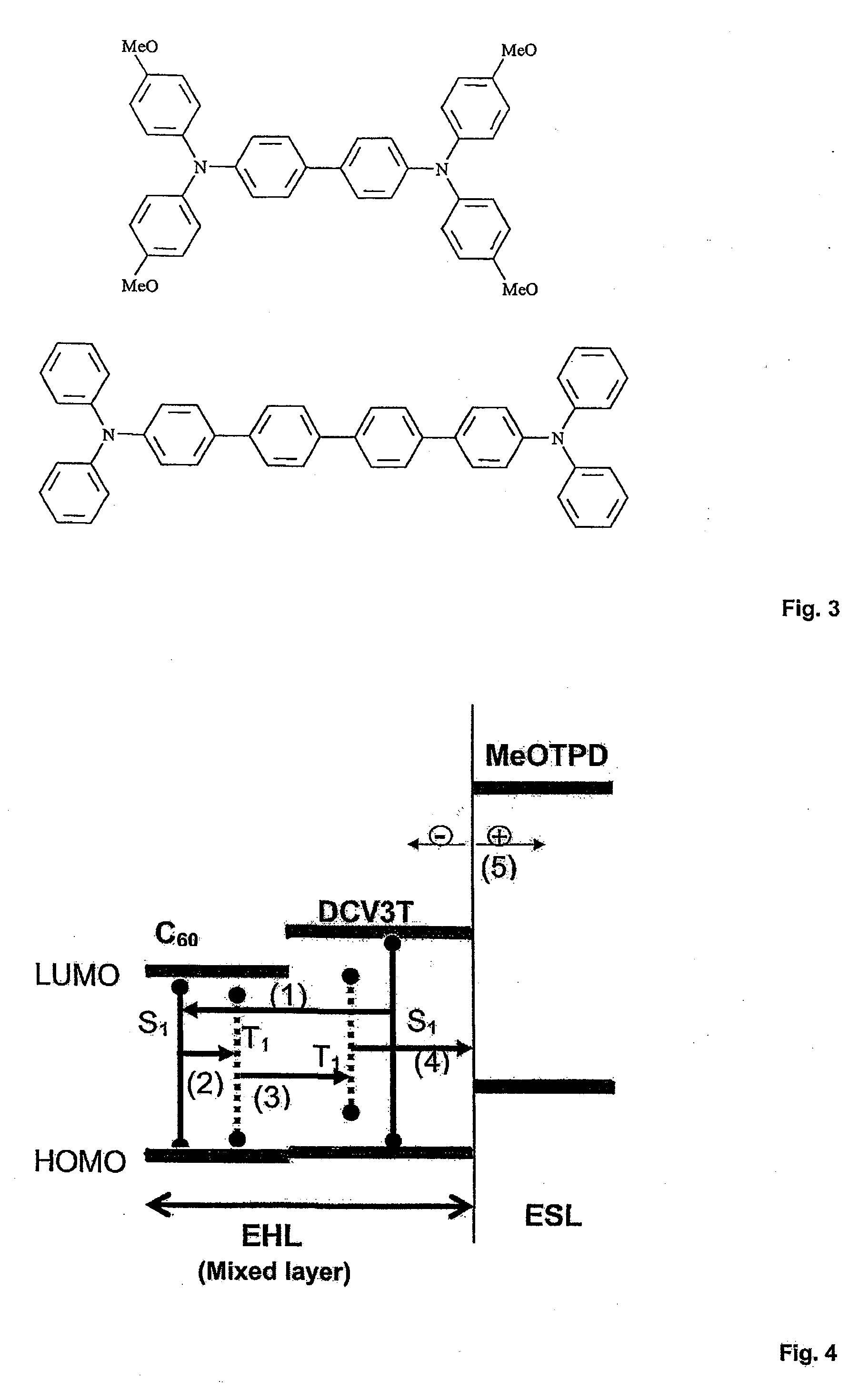
- A photoactive device with a layer arrangement featuring an exciton-harvesting layer and an exciton-separating layer, where the exciton-harvesting layer is a mixed layer of organic materials A and B, with material B converting singlet excitons to triplet excitons via inter-system crossing, and the exciton-separating layer facilitates the conversion of triplet excitons into free charge carriers, optimizing energy transfer and carrier separation.
Environmental Impact Assessment of Photoactive Compounds
The environmental implications of optimizing photoactive compounds for triplet yields above 0.6 present a complex landscape of both opportunities and challenges. Enhanced triplet formation efficiency directly correlates with improved photocatalytic performance, potentially reducing the overall material consumption required for environmental remediation applications. Higher triplet yields enable more effective pollutant degradation, water purification, and air treatment processes, thereby contributing to environmental protection efforts.
Manufacturing processes for high-efficiency photoactive compounds require careful consideration of resource utilization and waste generation. The synthesis of compounds with optimized triplet yields often involves precious metal catalysts, rare earth elements, or complex organic frameworks that may pose supply chain sustainability concerns. However, the improved efficiency of these materials can offset their environmental footprint through reduced operational energy requirements and extended service lifespans.
Lifecycle assessment studies indicate that photoactive compounds with enhanced triplet yields demonstrate superior environmental profiles when evaluated across their entire operational period. The increased quantum efficiency translates to reduced energy consumption per unit of photochemical conversion, lowering the carbon footprint of photocatalytic systems. This efficiency gain becomes particularly significant in large-scale applications such as industrial wastewater treatment or atmospheric pollutant removal.
End-of-life considerations for optimized photoactive compounds reveal both challenges and opportunities. While some high-performance materials may contain elements requiring specialized disposal protocols, their enhanced durability and efficiency often result in longer operational lifespans, reducing replacement frequency. Additionally, many photoactive compounds designed for high triplet yields incorporate recyclable components or can be regenerated through thermal or chemical treatments.
The deployment of optimized photoactive compounds in environmental applications demonstrates measurable benefits in terms of reduced chemical additive requirements and minimized secondary waste generation. These materials enable more selective photochemical processes, reducing the formation of unwanted byproducts that could pose additional environmental risks. Furthermore, their enhanced performance characteristics support the development of more compact and efficient treatment systems, reducing infrastructure requirements and associated environmental impacts.
Manufacturing processes for high-efficiency photoactive compounds require careful consideration of resource utilization and waste generation. The synthesis of compounds with optimized triplet yields often involves precious metal catalysts, rare earth elements, or complex organic frameworks that may pose supply chain sustainability concerns. However, the improved efficiency of these materials can offset their environmental footprint through reduced operational energy requirements and extended service lifespans.
Lifecycle assessment studies indicate that photoactive compounds with enhanced triplet yields demonstrate superior environmental profiles when evaluated across their entire operational period. The increased quantum efficiency translates to reduced energy consumption per unit of photochemical conversion, lowering the carbon footprint of photocatalytic systems. This efficiency gain becomes particularly significant in large-scale applications such as industrial wastewater treatment or atmospheric pollutant removal.
End-of-life considerations for optimized photoactive compounds reveal both challenges and opportunities. While some high-performance materials may contain elements requiring specialized disposal protocols, their enhanced durability and efficiency often result in longer operational lifespans, reducing replacement frequency. Additionally, many photoactive compounds designed for high triplet yields incorporate recyclable components or can be regenerated through thermal or chemical treatments.
The deployment of optimized photoactive compounds in environmental applications demonstrates measurable benefits in terms of reduced chemical additive requirements and minimized secondary waste generation. These materials enable more selective photochemical processes, reducing the formation of unwanted byproducts that could pose additional environmental risks. Furthermore, their enhanced performance characteristics support the development of more compact and efficient treatment systems, reducing infrastructure requirements and associated environmental impacts.
Safety Protocols for High-Efficiency Photosensitizer Development
The development of high-efficiency photosensitizers with triplet yields exceeding 0.6 necessitates comprehensive safety protocols to mitigate potential risks associated with enhanced photochemical activity. These compounds exhibit significantly increased reactivity under light exposure, requiring specialized handling procedures to prevent uncontrolled photochemical reactions that could lead to thermal runaway, toxic byproduct formation, or equipment damage.
Primary safety considerations include establishing controlled lighting environments throughout all development phases. Laboratory spaces must implement amber or red lighting systems with wavelengths above 600 nm to minimize inadvertent activation of photosensitizers during synthesis and characterization. Light-tight storage containers constructed from UV-blocking materials are essential for maintaining compound stability and preventing accidental exposure.
Personnel protection protocols require specialized training in photochemical hazards and mandatory use of UV-protective eyewear and clothing. Skin contact prevention becomes critical as high-efficiency photosensitizers can cause severe photosensitization reactions even at low concentrations. Emergency procedures must include immediate dark environment protocols and specialized decontamination methods for both personnel and equipment exposure incidents.
Synthesis safety measures encompass inert atmosphere requirements, temperature monitoring systems, and automated light exposure controls. Reaction vessels must incorporate pressure relief systems to handle potential gas evolution from enhanced triplet state reactions. Continuous monitoring of oxygen levels is crucial since high triplet yields can lead to increased singlet oxygen generation, creating fire and explosion hazards.
Analytical characterization safety involves implementing remote measurement capabilities for photophysical property determination. Laser safety protocols become paramount when conducting triplet yield measurements, requiring Class IV laser safety procedures and appropriate beam containment systems. Sample preparation must occur under controlled conditions with minimal light exposure to prevent degradation or unwanted photochemical transformations.
Waste disposal protocols require specialized procedures for photosensitive materials, including light-protected collection systems and approved disposal methods that account for persistent photochemical activity. Environmental release prevention measures must address both the parent compounds and potential photodegradation products that may retain biological activity.
Primary safety considerations include establishing controlled lighting environments throughout all development phases. Laboratory spaces must implement amber or red lighting systems with wavelengths above 600 nm to minimize inadvertent activation of photosensitizers during synthesis and characterization. Light-tight storage containers constructed from UV-blocking materials are essential for maintaining compound stability and preventing accidental exposure.
Personnel protection protocols require specialized training in photochemical hazards and mandatory use of UV-protective eyewear and clothing. Skin contact prevention becomes critical as high-efficiency photosensitizers can cause severe photosensitization reactions even at low concentrations. Emergency procedures must include immediate dark environment protocols and specialized decontamination methods for both personnel and equipment exposure incidents.
Synthesis safety measures encompass inert atmosphere requirements, temperature monitoring systems, and automated light exposure controls. Reaction vessels must incorporate pressure relief systems to handle potential gas evolution from enhanced triplet state reactions. Continuous monitoring of oxygen levels is crucial since high triplet yields can lead to increased singlet oxygen generation, creating fire and explosion hazards.
Analytical characterization safety involves implementing remote measurement capabilities for photophysical property determination. Laser safety protocols become paramount when conducting triplet yield measurements, requiring Class IV laser safety procedures and appropriate beam containment systems. Sample preparation must occur under controlled conditions with minimal light exposure to prevent degradation or unwanted photochemical transformations.
Waste disposal protocols require specialized procedures for photosensitive materials, including light-protected collection systems and approved disposal methods that account for persistent photochemical activity. Environmental release prevention measures must address both the parent compounds and potential photodegradation products that may retain biological activity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!