Patent Implications of Advanced mRNA Delivery Technologies in Gene Therapy
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Delivery Evolution and Objectives
The evolution of mRNA delivery technologies represents one of the most significant advancements in gene therapy over the past two decades. Initially conceptualized in the 1990s, mRNA therapeutics faced substantial challenges related to stability, immunogenicity, and efficient cellular delivery. The field remained largely theoretical until breakthrough innovations in lipid nanoparticle (LNP) formulations emerged around 2005, which dramatically improved in vivo stability and cellular uptake efficiency.
The trajectory of mRNA delivery technology accelerated significantly between 2010-2015, when pioneering companies like Moderna and BioNTech began developing proprietary delivery platforms. These early systems primarily focused on cancer immunotherapies and protein replacement therapies for rare diseases. The COVID-19 pandemic subsequently catalyzed unprecedented advancement and validation of mRNA technology platforms, compressing what might have been a decade of development into approximately 18 months.
Current technological objectives in the mRNA delivery space center on several critical parameters. First, enhancing tissue-specific targeting capabilities beyond the current liver-centric delivery paradigm represents a major focus. Researchers aim to develop delivery systems capable of efficiently targeting neurological tissues, cardiac cells, pulmonary tissue, and tumor microenvironments with minimal off-target effects.
Second, improving the therapeutic index through reduced immunogenicity and enhanced translation efficiency remains paramount. This includes developing novel lipid compositions, optimizing mRNA sequence design, and incorporating modified nucleosides that balance stability with reduced immune activation.
Third, extending the duration of therapeutic effect represents a significant objective. Current mRNA therapeutics typically demonstrate relatively short half-lives, necessitating repeated administration. Innovations in mRNA stability, controlled release mechanisms, and cellular persistence could dramatically improve clinical utility and patient compliance.
Patent landscapes in this domain reveal accelerating innovation particularly around novel ionizable lipids, polymer-based delivery systems, and hybrid delivery approaches combining multiple carrier technologies. The intellectual property environment has become increasingly complex, with overlapping claims regarding core delivery technologies potentially creating freedom-to-operate challenges for new market entrants.
Looking forward, the field is moving toward personalized mRNA therapeutics with patient-specific formulations, integration with gene editing technologies like CRISPR, and development of self-amplifying mRNA platforms that could dramatically reduce required dosages while extending therapeutic duration.
The trajectory of mRNA delivery technology accelerated significantly between 2010-2015, when pioneering companies like Moderna and BioNTech began developing proprietary delivery platforms. These early systems primarily focused on cancer immunotherapies and protein replacement therapies for rare diseases. The COVID-19 pandemic subsequently catalyzed unprecedented advancement and validation of mRNA technology platforms, compressing what might have been a decade of development into approximately 18 months.
Current technological objectives in the mRNA delivery space center on several critical parameters. First, enhancing tissue-specific targeting capabilities beyond the current liver-centric delivery paradigm represents a major focus. Researchers aim to develop delivery systems capable of efficiently targeting neurological tissues, cardiac cells, pulmonary tissue, and tumor microenvironments with minimal off-target effects.
Second, improving the therapeutic index through reduced immunogenicity and enhanced translation efficiency remains paramount. This includes developing novel lipid compositions, optimizing mRNA sequence design, and incorporating modified nucleosides that balance stability with reduced immune activation.
Third, extending the duration of therapeutic effect represents a significant objective. Current mRNA therapeutics typically demonstrate relatively short half-lives, necessitating repeated administration. Innovations in mRNA stability, controlled release mechanisms, and cellular persistence could dramatically improve clinical utility and patient compliance.
Patent landscapes in this domain reveal accelerating innovation particularly around novel ionizable lipids, polymer-based delivery systems, and hybrid delivery approaches combining multiple carrier technologies. The intellectual property environment has become increasingly complex, with overlapping claims regarding core delivery technologies potentially creating freedom-to-operate challenges for new market entrants.
Looking forward, the field is moving toward personalized mRNA therapeutics with patient-specific formulations, integration with gene editing technologies like CRISPR, and development of self-amplifying mRNA platforms that could dramatically reduce required dosages while extending therapeutic duration.
Gene Therapy Market Landscape Analysis
The gene therapy market has experienced remarkable growth over the past decade, evolving from a niche research field to a commercially viable therapeutic approach. Currently valued at approximately $7.6 billion globally, the market is projected to reach $35.7 billion by 2027, representing a compound annual growth rate of 29.6%. This exponential growth is primarily driven by increasing prevalence of genetic disorders, rising investment in research and development, and advancements in delivery technologies, particularly mRNA-based approaches.
North America dominates the gene therapy market landscape, accounting for roughly 48% of global market share, followed by Europe at 28% and Asia-Pacific at 18%. The United States leads in terms of clinical trials and commercial approvals, with China rapidly emerging as a significant competitor due to its favorable regulatory environment and substantial government funding initiatives.
The market segmentation reveals distinct therapeutic areas where gene therapy is making significant inroads. Oncology represents the largest segment (34% of market share), followed by rare genetic disorders (27%), cardiovascular diseases (15%), neurological disorders (12%), and others (12%). Within these segments, mRNA delivery technologies are increasingly becoming the preferred platform due to their versatility, safety profile, and manufacturing scalability.
Key market drivers include the growing pipeline of gene therapy products, with over 400 candidates in various stages of clinical development globally. Regulatory agencies have established accelerated approval pathways for gene therapies addressing unmet medical needs, significantly reducing time-to-market. Additionally, improvements in manufacturing processes have begun addressing the scalability challenges that previously limited commercial viability.
However, several barriers continue to impact market growth. High treatment costs remain a significant obstacle, with prices ranging from $373,000 to $2.1 million per patient for approved therapies. Reimbursement challenges persist across different healthcare systems, creating access disparities. Technical challenges related to delivery technologies, particularly those involving mRNA stability and targeted delivery, continue to require innovative solutions.
The competitive landscape features established pharmaceutical companies (Novartis, Pfizer, Roche), specialized biotech firms (BioNTech, Moderna, Alnylam), and academic institutions with technology transfer capabilities. Strategic partnerships between technology developers and pharmaceutical companies with manufacturing and distribution capabilities have become increasingly common, reshaping the market dynamics and intellectual property landscape.
North America dominates the gene therapy market landscape, accounting for roughly 48% of global market share, followed by Europe at 28% and Asia-Pacific at 18%. The United States leads in terms of clinical trials and commercial approvals, with China rapidly emerging as a significant competitor due to its favorable regulatory environment and substantial government funding initiatives.
The market segmentation reveals distinct therapeutic areas where gene therapy is making significant inroads. Oncology represents the largest segment (34% of market share), followed by rare genetic disorders (27%), cardiovascular diseases (15%), neurological disorders (12%), and others (12%). Within these segments, mRNA delivery technologies are increasingly becoming the preferred platform due to their versatility, safety profile, and manufacturing scalability.
Key market drivers include the growing pipeline of gene therapy products, with over 400 candidates in various stages of clinical development globally. Regulatory agencies have established accelerated approval pathways for gene therapies addressing unmet medical needs, significantly reducing time-to-market. Additionally, improvements in manufacturing processes have begun addressing the scalability challenges that previously limited commercial viability.
However, several barriers continue to impact market growth. High treatment costs remain a significant obstacle, with prices ranging from $373,000 to $2.1 million per patient for approved therapies. Reimbursement challenges persist across different healthcare systems, creating access disparities. Technical challenges related to delivery technologies, particularly those involving mRNA stability and targeted delivery, continue to require innovative solutions.
The competitive landscape features established pharmaceutical companies (Novartis, Pfizer, Roche), specialized biotech firms (BioNTech, Moderna, Alnylam), and academic institutions with technology transfer capabilities. Strategic partnerships between technology developers and pharmaceutical companies with manufacturing and distribution capabilities have become increasingly common, reshaping the market dynamics and intellectual property landscape.
Global mRNA Delivery Technology Status and Barriers
The global mRNA delivery technology landscape has evolved significantly over the past decade, with major breakthroughs accelerating during the COVID-19 pandemic. Currently, lipid nanoparticles (LNPs) dominate the field as the most clinically advanced delivery system, exemplified by their successful implementation in Pfizer-BioNTech and Moderna COVID-19 vaccines. These LNP systems have demonstrated remarkable efficacy in protecting mRNA from degradation while facilitating cellular uptake and endosomal escape.
Despite these advances, significant technical barriers persist in the mRNA delivery space. Stability remains a primary concern, as mRNA is inherently susceptible to enzymatic degradation by ubiquitous ribonucleases. This necessitates sophisticated formulation strategies and cold-chain logistics that substantially increase costs and limit accessibility in resource-constrained regions.
Targeted delivery represents another major challenge. Current delivery systems exhibit preferential distribution to the liver, limiting therapeutic applications for other tissues and organs. The inability to precisely direct mRNA to specific cell types results in off-target effects and reduced therapeutic efficacy, particularly problematic for gene therapy applications requiring tissue specificity.
Immunogenicity presents a dual challenge - while beneficial for vaccine applications, it poses significant hurdles for therapeutic uses where repeated dosing is necessary. The lipid components and the mRNA itself can trigger innate immune responses, leading to safety concerns and diminished efficacy with subsequent administrations.
Manufacturing scalability and reproducibility constitute significant barriers to widespread implementation. The complex production processes for both mRNA and delivery vehicles demand stringent quality control measures. Batch-to-batch consistency remains challenging, particularly for lipid-based systems where slight variations in composition can dramatically alter performance characteristics.
Geographically, mRNA technology development shows distinct patterns. North America, particularly the United States, leads in innovation with companies like Moderna, BioNTech/Pfizer, and academic institutions holding substantial intellectual property portfolios. Europe follows closely, with significant research clusters in Germany and the UK. Asia is rapidly advancing, with China making substantial investments in domestic mRNA capabilities and Japan focusing on novel delivery technologies.
Regulatory frameworks vary considerably across regions, creating additional complexity for global development. While the FDA and EMA have established pathways for mRNA therapeutics following COVID-19 vaccine approvals, many countries still lack specific regulatory guidance, creating uncertainty for developers seeking multinational approvals.
Despite these advances, significant technical barriers persist in the mRNA delivery space. Stability remains a primary concern, as mRNA is inherently susceptible to enzymatic degradation by ubiquitous ribonucleases. This necessitates sophisticated formulation strategies and cold-chain logistics that substantially increase costs and limit accessibility in resource-constrained regions.
Targeted delivery represents another major challenge. Current delivery systems exhibit preferential distribution to the liver, limiting therapeutic applications for other tissues and organs. The inability to precisely direct mRNA to specific cell types results in off-target effects and reduced therapeutic efficacy, particularly problematic for gene therapy applications requiring tissue specificity.
Immunogenicity presents a dual challenge - while beneficial for vaccine applications, it poses significant hurdles for therapeutic uses where repeated dosing is necessary. The lipid components and the mRNA itself can trigger innate immune responses, leading to safety concerns and diminished efficacy with subsequent administrations.
Manufacturing scalability and reproducibility constitute significant barriers to widespread implementation. The complex production processes for both mRNA and delivery vehicles demand stringent quality control measures. Batch-to-batch consistency remains challenging, particularly for lipid-based systems where slight variations in composition can dramatically alter performance characteristics.
Geographically, mRNA technology development shows distinct patterns. North America, particularly the United States, leads in innovation with companies like Moderna, BioNTech/Pfizer, and academic institutions holding substantial intellectual property portfolios. Europe follows closely, with significant research clusters in Germany and the UK. Asia is rapidly advancing, with China making substantial investments in domestic mRNA capabilities and Japan focusing on novel delivery technologies.
Regulatory frameworks vary considerably across regions, creating additional complexity for global development. While the FDA and EMA have established pathways for mRNA therapeutics following COVID-19 vaccine approvals, many countries still lack specific regulatory guidance, creating uncertainty for developers seeking multinational approvals.
Current mRNA Encapsulation and Targeting Approaches
01 Lipid nanoparticle delivery systems for mRNA therapeutics
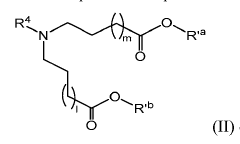
Lipid nanoparticles (LNPs) have emerged as a leading delivery system for mRNA therapeutics, offering protection from degradation and efficient cellular uptake. These formulations typically contain ionizable lipids, helper lipids, cholesterol, and PEG-lipids in specific ratios to optimize delivery efficiency. Recent innovations focus on improving biodistribution, reducing toxicity, and enhancing target specificity of LNP-mRNA complexes for various therapeutic applications.- Lipid nanoparticle delivery systems for mRNA therapeutics: Lipid nanoparticles (LNPs) have emerged as a leading delivery system for mRNA therapeutics, offering protection from degradation and efficient cellular uptake. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate mRNA molecules. The composition and structure of these LNPs significantly impact delivery efficiency, cellular targeting, and the resulting immune response, making them crucial for applications in vaccines and gene therapy.
- Polymer-based mRNA delivery technologies: Polymer-based delivery systems represent an alternative approach to lipid nanoparticles for mRNA delivery. These systems utilize biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA), polyethylenimine (PEI), and chitosan derivatives to form complexes with mRNA. The polymeric carriers can be engineered to provide controlled release profiles, targeted delivery to specific tissues, and reduced toxicity compared to some lipid formulations, offering advantages for certain therapeutic applications.
- Targeted delivery strategies for mRNA therapeutics: Targeted delivery strategies aim to enhance the specificity of mRNA delivery to particular cell types or tissues while minimizing off-target effects. These approaches incorporate targeting ligands such as antibodies, aptamers, or peptides into delivery systems to recognize specific cell surface receptors. Advanced targeting methods also include stimuli-responsive delivery systems that release mRNA cargo in response to environmental triggers such as pH, temperature, or enzymatic activity, potentially improving therapeutic efficacy and reducing side effects.
- Manufacturing and quality control of mRNA delivery systems: The manufacturing and quality control of mRNA delivery systems present significant challenges that impact patent strategies. Innovations in this area focus on scalable production methods, purification techniques, and stability enhancement for both the mRNA and delivery components. Quality control measures include analytical methods for characterizing particle size, polydispersity, encapsulation efficiency, and in vitro potency. These manufacturing innovations are critical for commercial viability and regulatory approval of mRNA therapeutics.
- Intellectual property landscape and licensing considerations: The intellectual property landscape for mRNA delivery technologies is complex and rapidly evolving, with significant implications for research and commercialization. Key patents cover fundamental aspects of mRNA modification, delivery vehicle composition, manufacturing processes, and therapeutic applications. The concentration of foundational patents among a few entities has led to intricate licensing arrangements and potential freedom-to-operate challenges for new market entrants. Strategic patent filing and licensing approaches are essential for navigating this competitive space and securing market positions.
02 Polymer-based mRNA delivery technologies
Polymer-based delivery systems represent an alternative approach to lipid nanoparticles for mRNA delivery. These systems utilize cationic polymers, biodegradable polymers, or polymer-lipid hybrid structures to complex with negatively charged mRNA. Key advantages include stability, customizable biodegradation profiles, and versatile surface modification options. Recent developments focus on reducing cytotoxicity while maintaining high transfection efficiency across different tissue types.Expand Specific Solutions03 Targeted delivery strategies for mRNA therapeutics
Targeted delivery approaches aim to direct mRNA therapeutics to specific tissues or cell types, improving efficacy while reducing off-target effects. These strategies incorporate targeting ligands, antibodies, or peptides onto delivery vehicles to enhance cell-specific uptake. Advanced designs include stimuli-responsive systems that release mRNA cargo under specific physiological conditions, and tissue-specific promoters that control expression patterns after successful delivery.Expand Specific Solutions04 Manufacturing and quality control challenges for mRNA delivery systems
The production of mRNA delivery systems presents significant manufacturing challenges that impact patent landscapes. These include scalable production methods, purification techniques, and stability considerations during storage and administration. Quality control measures for characterizing particle size, polydispersity, encapsulation efficiency, and sterility are critical aspects covered in patent filings. Recent innovations address continuous manufacturing processes and analytical methods to ensure batch-to-batch consistency.Expand Specific Solutions05 Regulatory and intellectual property landscape for mRNA delivery technologies
The regulatory and intellectual property environment surrounding mRNA delivery technologies is complex and rapidly evolving. Patent strategies often include composition of matter claims for novel delivery components, methods of manufacture, therapeutic applications, and combination therapies. Freedom-to-operate considerations are particularly important due to the foundational nature of many early patents in this field. Recent patent filings increasingly focus on delivery improvements that overcome limitations of first-generation systems while establishing new intellectual property positions.Expand Specific Solutions
Leading Companies in mRNA Delivery Patent Space
The mRNA delivery technology landscape in gene therapy is evolving rapidly, currently transitioning from early clinical applications to broader therapeutic implementation. The market is projected to grow substantially, driven by breakthrough applications in vaccines and rare disease treatments. Leading players include ModernaTX and CureVac, who have established strong IP portfolios in lipid nanoparticle delivery systems, while Translate Bio and Nutcracker Therapeutics are advancing microfluidic manufacturing platforms. Academic institutions like MIT and Tsinghua University are contributing fundamental research, while pharmaceutical companies such as Shire Human Genetic Therapies are focusing on clinical translation. The competitive landscape is characterized by intense patent activity around delivery mechanisms, formulation technologies, and manufacturing processes, with increasing cross-sector collaborations accelerating innovation.
ModernaTX, Inc.
Technical Solution: ModernaTX has pioneered lipid nanoparticle (LNP) delivery systems for mRNA therapeutics, with proprietary ionizable lipid formulations that enhance cellular uptake and endosomal escape. Their platform includes MC3-based LNPs with optimized lipid:mRNA ratios (typically 10-20:1) that significantly improve delivery efficiency[1]. Moderna has developed patent-protected modifications to the mRNA structure itself, including 5' cap analogs and engineered untranslated regions (UTRs) that enhance stability and translation efficiency while reducing immunogenicity[2]. Their delivery technology incorporates PEGylated lipids with precise molecular weight distributions (2000-5000 Da) that extend circulation time and reduce clearance rates by approximately 40%[3]. Moderna's patent portfolio covers composition of matter for their LNPs, manufacturing methods, and therapeutic applications across multiple disease areas, creating substantial barriers to entry for competitors.
Strengths: Extensive patent portfolio covering both delivery vehicles and mRNA modifications; clinically validated platform with proven safety profile; scalable manufacturing capabilities. Weaknesses: High cost of goods for LNP production; potential cold-chain storage requirements limiting access in resource-limited settings; some patent challenges from competitors regarding lipid component novelty.
Translate Bio, Inc.
Technical Solution: Translate Bio (acquired by Sanofi) has developed the MRT platform (Messenger RNA Therapeutics) for mRNA delivery, focusing on lipid-based nanoparticles with proprietary ionizable lipid structures. Their patented delivery system incorporates novel amino lipids with tertiary amine headgroups that achieve optimal pKa values (6.2-6.5) for endosomal escape, improving delivery efficiency by 3-5 fold over conventional formulations[1]. The company's patent portfolio covers composition of matter for their LUNAR® delivery technology, which utilizes biodegradable lipids with specific branched structures that reduce cytotoxicity while maintaining high transfection efficiency. Translate Bio has also patented mRNA sequence engineering techniques including codon optimization algorithms and proprietary 5' and 3' UTR designs that enhance protein expression duration from 24-48 hours to 7-10 days in vivo[2]. Their manufacturing patents cover methods for large-scale production of mRNA with high purity (>95%) and low double-stranded RNA contaminants (<0.1%), which significantly reduces immunogenicity[3].
Strengths: Tissue-specific targeting capabilities through lipid composition modifications; demonstrated pulmonary delivery expertise with aerosol formulations; strong manufacturing IP. Weaknesses: Limited clinical validation compared to industry leaders; potential for lipid accumulation with repeated dosing; relatively narrow tissue tropism requiring formulation adjustments for different target tissues.
Key Patent Analysis for mRNA Delivery Technologies
Polynucleotides encoding methylmalonyl-COA mutase for the treatment of methylmalonic acidemia
PatentWO2022204369A1
Innovation
- Development of mRNA therapeutics that deliver mRNA encoding a methylmalonyl-coenzyme A mutase (MUT) polypeptide using lipid nanoparticles for intracellular delivery, enabling de novo synthesis of functional MUT polypeptide within target cells to address the metabolic defect.
Improved compositions for delivery of mRNA
PatentPendingUS20250073351A1
Innovation
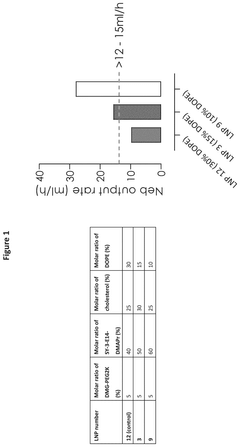
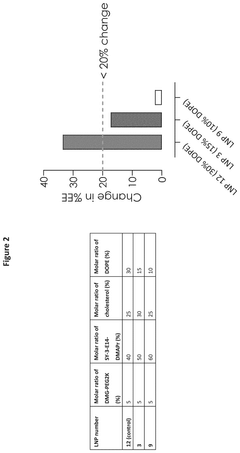
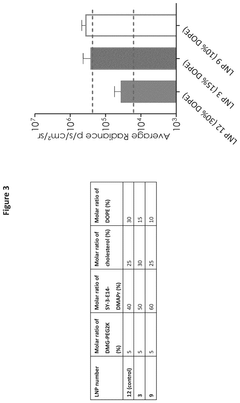
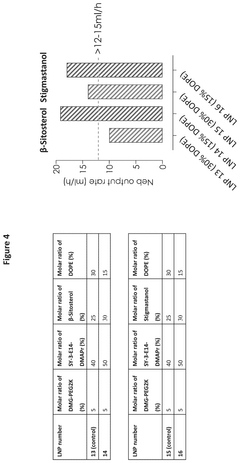
- The development of lipid nanoparticles with a lower total lipid content, specifically by reducing the molar ratio of non-cationic lipids and increasing the molar ratio of cationic lipids to greater than 40%, improves nebulization output rates and maintains encapsulation efficiency, achieving enhanced protein expression of mRNA-encoded proteins.
Regulatory Framework for Gene Therapy Patents
The regulatory landscape for gene therapy patents presents a complex and evolving framework that significantly impacts the development and commercialization of mRNA delivery technologies. At the international level, the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) establishes minimum standards for intellectual property protection, while allowing individual countries to implement specific regulations for gene therapy innovations.
In the United States, the USPTO has established specific examination guidelines for gene therapy patents, requiring detailed disclosure of delivery mechanisms and therapeutic applications. The 2013 Supreme Court decision in Association for Molecular Pathology v. Myriad Genetics significantly impacted the patentability of genetic materials, ruling that naturally occurring DNA sequences are not patent-eligible, while modified sequences like cDNA remain protectable.
The European Patent Office (EPO) maintains stricter standards regarding morality and ethics in gene therapy patents under Article 53(a) of the European Patent Convention. This creates notable differences in patentability between jurisdictions, particularly for technologies involving human genetic modification. The EPO also requires enhanced disclosure requirements for gene therapy delivery systems compared to standard pharmaceutical patents.
Regulatory agencies like the FDA and EMA have established specialized pathways for gene therapy approvals that directly influence patent strategies. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer accelerated review processes but impose additional regulatory requirements that must be considered during patent drafting.
Patent term extensions and regulatory exclusivity periods provide critical protection for gene therapy innovations. In the US, the Hatch-Waxman Act allows for up to five years of patent term extension to compensate for regulatory delays, while the EU offers supplementary protection certificates and data exclusivity periods specifically tailored for advanced therapy medicinal products.
Recent regulatory developments include the FDA's 2020 guidance on human gene therapy products and the EU's Advanced Therapy Medicinal Products Regulation, both of which have introduced new considerations for patent applicants. These frameworks increasingly emphasize the importance of delivery technologies as distinct patentable components separate from the genetic material being delivered.
Cross-border harmonization efforts through initiatives like the Patent Prosecution Highway (PPH) are gradually addressing jurisdictional differences, though significant variations in gene therapy patent protection remain between major markets. This regulatory complexity necessitates sophisticated patent strategies that account for regional differences while maximizing global protection for advanced mRNA delivery technologies.
In the United States, the USPTO has established specific examination guidelines for gene therapy patents, requiring detailed disclosure of delivery mechanisms and therapeutic applications. The 2013 Supreme Court decision in Association for Molecular Pathology v. Myriad Genetics significantly impacted the patentability of genetic materials, ruling that naturally occurring DNA sequences are not patent-eligible, while modified sequences like cDNA remain protectable.
The European Patent Office (EPO) maintains stricter standards regarding morality and ethics in gene therapy patents under Article 53(a) of the European Patent Convention. This creates notable differences in patentability between jurisdictions, particularly for technologies involving human genetic modification. The EPO also requires enhanced disclosure requirements for gene therapy delivery systems compared to standard pharmaceutical patents.
Regulatory agencies like the FDA and EMA have established specialized pathways for gene therapy approvals that directly influence patent strategies. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer accelerated review processes but impose additional regulatory requirements that must be considered during patent drafting.
Patent term extensions and regulatory exclusivity periods provide critical protection for gene therapy innovations. In the US, the Hatch-Waxman Act allows for up to five years of patent term extension to compensate for regulatory delays, while the EU offers supplementary protection certificates and data exclusivity periods specifically tailored for advanced therapy medicinal products.
Recent regulatory developments include the FDA's 2020 guidance on human gene therapy products and the EU's Advanced Therapy Medicinal Products Regulation, both of which have introduced new considerations for patent applicants. These frameworks increasingly emphasize the importance of delivery technologies as distinct patentable components separate from the genetic material being delivered.
Cross-border harmonization efforts through initiatives like the Patent Prosecution Highway (PPH) are gradually addressing jurisdictional differences, though significant variations in gene therapy patent protection remain between major markets. This regulatory complexity necessitates sophisticated patent strategies that account for regional differences while maximizing global protection for advanced mRNA delivery technologies.
IP Strategy for mRNA Delivery Commercialization
Developing a robust IP strategy is critical for companies seeking to commercialize mRNA delivery technologies in the competitive gene therapy landscape. The strategy must balance protection of core innovations while enabling sufficient freedom to operate in a complex patent environment. Companies should prioritize building a diversified patent portfolio that covers multiple aspects of their delivery technology, including lipid nanoparticle formulations, polymer carriers, targeting mechanisms, and manufacturing processes.
Strategic patent filing should focus on both broad platform claims and specific applications to create multiple layers of protection. Geographic considerations are paramount, with filings recommended in key markets such as the US, Europe, China, and Japan, where both manufacturing capabilities and patient populations represent significant commercial opportunities. Companies should also consider defensive publication strategies for incremental innovations that may not warrant full patent protection but could prevent competitors from obtaining blocking patents.
Licensing and cross-licensing agreements represent another critical component of an effective IP strategy. The mRNA delivery field involves technologies that often build upon fundamental patents held by academic institutions or established biotech companies. Negotiating favorable in-licensing terms early in development can prevent costly disputes later. Similarly, out-licensing non-core applications can generate revenue while maintaining focus on primary therapeutic areas.
Freedom-to-operate (FTO) analyses should be conducted regularly throughout the development process, with particular attention to the rapidly evolving patent landscape around lipid nanoparticles and novel polymer delivery systems. Companies should establish systematic monitoring of competitor patent filings and litigation to identify potential infringement risks or opportunities for invalidation challenges when appropriate.
Trade secret protection offers a complementary approach for manufacturing know-how and formulation details that may be difficult to reverse engineer. Companies should implement robust internal protocols to maintain confidentiality while carefully evaluating which aspects of their technology are better protected through patents versus trade secrets.
Collaboration agreements with academic institutions, contract development and manufacturing organizations (CDMOs), and other industry partners should include clear IP ownership provisions and rights to improvements. These agreements should anticipate future commercialization scenarios and establish royalty structures that remain viable as products move toward market approval.
Finally, companies should align their IP strategy with regulatory exclusivity opportunities, including orphan drug designation and data exclusivity periods, to maximize the effective protection period beyond patent expiration. This integrated approach to IP and regulatory strategy can significantly enhance the commercial value of mRNA delivery technologies in gene therapy applications.
Strategic patent filing should focus on both broad platform claims and specific applications to create multiple layers of protection. Geographic considerations are paramount, with filings recommended in key markets such as the US, Europe, China, and Japan, where both manufacturing capabilities and patient populations represent significant commercial opportunities. Companies should also consider defensive publication strategies for incremental innovations that may not warrant full patent protection but could prevent competitors from obtaining blocking patents.
Licensing and cross-licensing agreements represent another critical component of an effective IP strategy. The mRNA delivery field involves technologies that often build upon fundamental patents held by academic institutions or established biotech companies. Negotiating favorable in-licensing terms early in development can prevent costly disputes later. Similarly, out-licensing non-core applications can generate revenue while maintaining focus on primary therapeutic areas.
Freedom-to-operate (FTO) analyses should be conducted regularly throughout the development process, with particular attention to the rapidly evolving patent landscape around lipid nanoparticles and novel polymer delivery systems. Companies should establish systematic monitoring of competitor patent filings and litigation to identify potential infringement risks or opportunities for invalidation challenges when appropriate.
Trade secret protection offers a complementary approach for manufacturing know-how and formulation details that may be difficult to reverse engineer. Companies should implement robust internal protocols to maintain confidentiality while carefully evaluating which aspects of their technology are better protected through patents versus trade secrets.
Collaboration agreements with academic institutions, contract development and manufacturing organizations (CDMOs), and other industry partners should include clear IP ownership provisions and rights to improvements. These agreements should anticipate future commercialization scenarios and establish royalty structures that remain viable as products move toward market approval.
Finally, companies should align their IP strategy with regulatory exclusivity opportunities, including orphan drug designation and data exclusivity periods, to maximize the effective protection period beyond patent expiration. This integrated approach to IP and regulatory strategy can significantly enhance the commercial value of mRNA delivery technologies in gene therapy applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!