Select Photoactive Compound For Bioimaging With nM Detection Limits
DEC 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photoactive Compound Bioimaging Background and Objectives
Bioimaging has emerged as a cornerstone technology in modern biomedical research and clinical diagnostics, enabling real-time visualization of biological processes at cellular and molecular levels. The field has witnessed remarkable evolution from traditional fluorescent proteins to sophisticated photoactive compounds capable of detecting biomolecules at unprecedented sensitivity levels. This technological progression addresses the critical need for non-invasive, high-resolution imaging techniques that can monitor disease progression, drug distribution, and therapeutic efficacy in living systems.
The development of photoactive compounds for bioimaging represents a convergence of chemistry, physics, and biology, where molecular design principles are applied to create probes with enhanced optical properties. Historical milestones include the introduction of organic fluorophores in the 1960s, followed by quantum dots in the 1990s, and more recently, near-infrared fluorescent proteins and activatable probes. Each generation has pushed the boundaries of detection sensitivity, photostability, and biocompatibility.
Current technological trends emphasize the development of compounds capable of achieving nanomolar detection limits, representing a thousand-fold improvement over conventional imaging agents. This sensitivity threshold is crucial for detecting low-abundance biomarkers, monitoring early-stage disease processes, and tracking single-molecule events in complex biological environments. The pursuit of nanomolar sensitivity has driven innovations in molecular engineering, including the design of signal amplification mechanisms and background noise reduction strategies.
The primary objective of selecting optimal photoactive compounds centers on achieving reliable nanomolar detection while maintaining excellent signal-to-noise ratios and minimal cytotoxicity. Key performance metrics include quantum yield optimization, photostability under physiological conditions, and selective targeting capabilities. Additionally, compounds must demonstrate compatibility with existing imaging infrastructure and provide sufficient penetration depth for in vivo applications.
Future technological goals encompass the development of multiplexed imaging systems capable of simultaneous detection of multiple targets, real-time monitoring of dynamic biological processes, and integration with therapeutic modalities for theranostic applications. The ultimate vision involves creating a comprehensive toolkit of photoactive compounds that can address diverse imaging challenges across various biological scales and disease contexts.
The development of photoactive compounds for bioimaging represents a convergence of chemistry, physics, and biology, where molecular design principles are applied to create probes with enhanced optical properties. Historical milestones include the introduction of organic fluorophores in the 1960s, followed by quantum dots in the 1990s, and more recently, near-infrared fluorescent proteins and activatable probes. Each generation has pushed the boundaries of detection sensitivity, photostability, and biocompatibility.
Current technological trends emphasize the development of compounds capable of achieving nanomolar detection limits, representing a thousand-fold improvement over conventional imaging agents. This sensitivity threshold is crucial for detecting low-abundance biomarkers, monitoring early-stage disease processes, and tracking single-molecule events in complex biological environments. The pursuit of nanomolar sensitivity has driven innovations in molecular engineering, including the design of signal amplification mechanisms and background noise reduction strategies.
The primary objective of selecting optimal photoactive compounds centers on achieving reliable nanomolar detection while maintaining excellent signal-to-noise ratios and minimal cytotoxicity. Key performance metrics include quantum yield optimization, photostability under physiological conditions, and selective targeting capabilities. Additionally, compounds must demonstrate compatibility with existing imaging infrastructure and provide sufficient penetration depth for in vivo applications.
Future technological goals encompass the development of multiplexed imaging systems capable of simultaneous detection of multiple targets, real-time monitoring of dynamic biological processes, and integration with therapeutic modalities for theranostic applications. The ultimate vision involves creating a comprehensive toolkit of photoactive compounds that can address diverse imaging challenges across various biological scales and disease contexts.
Market Demand for Ultra-Sensitive Bioimaging Solutions
The global bioimaging market is experiencing unprecedented growth driven by the critical need for ultra-sensitive detection capabilities in medical diagnostics, drug discovery, and biological research. Healthcare systems worldwide are increasingly demanding imaging solutions that can detect molecular targets at nanomolar concentrations, enabling earlier disease detection and more precise therapeutic monitoring.
Cancer diagnostics represents the largest market segment driving demand for ultra-sensitive bioimaging technologies. Early-stage tumor detection requires imaging agents capable of identifying sparse biomarkers and circulating tumor cells present in extremely low concentrations. Current clinical imaging modalities often fail to detect malignancies until they reach advanced stages, creating substantial market opportunities for photoactive compounds with nanomolar sensitivity.
Pharmaceutical companies constitute another major demand driver, particularly in drug development pipelines where ultra-sensitive imaging enables real-time monitoring of drug distribution, target engagement, and therapeutic efficacy. The ability to track drug molecules and their metabolites at nanomolar levels significantly reduces development timelines and costs while improving success rates in clinical trials.
Neurological disorder research presents rapidly expanding market opportunities, as researchers require imaging tools capable of detecting protein aggregates, neurotransmitter changes, and cellular dysfunction at the earliest disease stages. Alzheimer's disease, Parkinson's disease, and other neurodegenerative conditions demand ultra-sensitive detection to enable preventive interventions before irreversible damage occurs.
The personalized medicine trend further amplifies market demand for ultra-sensitive bioimaging solutions. Precision therapeutics require detailed molecular profiling of individual patients, necessitating imaging technologies that can detect and quantify specific biomarkers with exceptional sensitivity and specificity.
Academic research institutions and biotechnology companies are increasingly investing in ultra-sensitive imaging capabilities to advance fundamental biological understanding and accelerate translational research. The growing emphasis on single-cell analysis and molecular-level biological processes creates sustained demand for photoactive compounds with nanomolar detection limits.
Regulatory agencies are also driving market demand by establishing more stringent requirements for drug safety and efficacy demonstrations, necessitating advanced imaging technologies that can provide comprehensive molecular-level data throughout the development process.
Cancer diagnostics represents the largest market segment driving demand for ultra-sensitive bioimaging technologies. Early-stage tumor detection requires imaging agents capable of identifying sparse biomarkers and circulating tumor cells present in extremely low concentrations. Current clinical imaging modalities often fail to detect malignancies until they reach advanced stages, creating substantial market opportunities for photoactive compounds with nanomolar sensitivity.
Pharmaceutical companies constitute another major demand driver, particularly in drug development pipelines where ultra-sensitive imaging enables real-time monitoring of drug distribution, target engagement, and therapeutic efficacy. The ability to track drug molecules and their metabolites at nanomolar levels significantly reduces development timelines and costs while improving success rates in clinical trials.
Neurological disorder research presents rapidly expanding market opportunities, as researchers require imaging tools capable of detecting protein aggregates, neurotransmitter changes, and cellular dysfunction at the earliest disease stages. Alzheimer's disease, Parkinson's disease, and other neurodegenerative conditions demand ultra-sensitive detection to enable preventive interventions before irreversible damage occurs.
The personalized medicine trend further amplifies market demand for ultra-sensitive bioimaging solutions. Precision therapeutics require detailed molecular profiling of individual patients, necessitating imaging technologies that can detect and quantify specific biomarkers with exceptional sensitivity and specificity.
Academic research institutions and biotechnology companies are increasingly investing in ultra-sensitive imaging capabilities to advance fundamental biological understanding and accelerate translational research. The growing emphasis on single-cell analysis and molecular-level biological processes creates sustained demand for photoactive compounds with nanomolar detection limits.
Regulatory agencies are also driving market demand by establishing more stringent requirements for drug safety and efficacy demonstrations, necessitating advanced imaging technologies that can provide comprehensive molecular-level data throughout the development process.
Current State of nM Detection in Photoactive Bioimaging
The field of photoactive compound-based bioimaging has achieved remarkable progress in reaching nanomolar detection sensitivity, driven by advances in fluorophore design, imaging instrumentation, and signal amplification strategies. Current state-of-the-art photoactive compounds for nM detection primarily encompass organic fluorophores, quantum dots, upconversion nanoparticles, and genetically encoded fluorescent proteins, each offering distinct advantages for specific bioimaging applications.
Organic fluorophores represent the most mature category, with rhodamine derivatives, cyanine dyes, and BODIPY compounds demonstrating exceptional quantum yields and photostability. Recent developments in through-bond energy transfer systems and aggregation-induced emission luminogens have pushed detection limits into the sub-nanomolar range. These compounds benefit from well-established synthesis protocols and extensive bioconjugation chemistry, enabling precise targeting of cellular components.
Quantum dots have emerged as powerful alternatives, offering superior brightness and photostability compared to traditional organic dyes. Silicon quantum dots and carbon dots have gained particular attention due to their biocompatibility and tunable emission properties. Their high extinction coefficients and resistance to photobleaching make them ideal for long-term imaging studies requiring sustained nM sensitivity.
Upconversion nanoparticles present unique advantages for deep tissue imaging applications, converting near-infrared excitation to visible emission while minimizing autofluorescence interference. Lanthanide-doped upconversion systems have demonstrated detection capabilities in the low nanomolar range, particularly valuable for in vivo applications where tissue penetration is critical.
Despite these advances, several technical challenges persist in achieving consistent nM detection. Photobleaching remains a significant limitation for organic fluorophores during extended imaging sessions. Background fluorescence from biological samples continues to impact signal-to-noise ratios, particularly in complex tissue environments. Additionally, the trade-off between brightness and biocompatibility often constrains the selection of optimal photoactive compounds for specific applications.
Current detection methodologies integrate advanced microscopy techniques including super-resolution imaging, time-gated detection, and machine learning-enhanced image processing to maximize sensitivity. Single-molecule detection capabilities have been demonstrated under controlled conditions, though practical biological applications still face reproducibility challenges. The integration of signal amplification strategies, such as enzymatic turnover and DNA amplification cascades, has successfully extended detection limits while maintaining specificity for target biomolecules.
Organic fluorophores represent the most mature category, with rhodamine derivatives, cyanine dyes, and BODIPY compounds demonstrating exceptional quantum yields and photostability. Recent developments in through-bond energy transfer systems and aggregation-induced emission luminogens have pushed detection limits into the sub-nanomolar range. These compounds benefit from well-established synthesis protocols and extensive bioconjugation chemistry, enabling precise targeting of cellular components.
Quantum dots have emerged as powerful alternatives, offering superior brightness and photostability compared to traditional organic dyes. Silicon quantum dots and carbon dots have gained particular attention due to their biocompatibility and tunable emission properties. Their high extinction coefficients and resistance to photobleaching make them ideal for long-term imaging studies requiring sustained nM sensitivity.
Upconversion nanoparticles present unique advantages for deep tissue imaging applications, converting near-infrared excitation to visible emission while minimizing autofluorescence interference. Lanthanide-doped upconversion systems have demonstrated detection capabilities in the low nanomolar range, particularly valuable for in vivo applications where tissue penetration is critical.
Despite these advances, several technical challenges persist in achieving consistent nM detection. Photobleaching remains a significant limitation for organic fluorophores during extended imaging sessions. Background fluorescence from biological samples continues to impact signal-to-noise ratios, particularly in complex tissue environments. Additionally, the trade-off between brightness and biocompatibility often constrains the selection of optimal photoactive compounds for specific applications.
Current detection methodologies integrate advanced microscopy techniques including super-resolution imaging, time-gated detection, and machine learning-enhanced image processing to maximize sensitivity. Single-molecule detection capabilities have been demonstrated under controlled conditions, though practical biological applications still face reproducibility challenges. The integration of signal amplification strategies, such as enzymatic turnover and DNA amplification cascades, has successfully extended detection limits while maintaining specificity for target biomolecules.
Existing Photoactive Compounds for nM Detection
01 Spectroscopic detection methods for photoactive compounds
Various spectroscopic techniques are employed to detect and quantify photoactive compounds at low concentrations. These methods utilize the optical properties of photoactive substances, including absorption, fluorescence, and luminescence characteristics. Advanced spectroscopic approaches can achieve high sensitivity and selectivity for different types of photoactive materials, enabling precise measurement of detection limits across various sample matrices.- Spectroscopic detection methods for photoactive compounds: Various spectroscopic techniques are employed to detect and quantify photoactive compounds with high sensitivity. These methods utilize light absorption, fluorescence, and other optical properties to identify compounds at very low concentrations. Advanced spectroscopic approaches can achieve detection limits in the nanogram to picogram range, making them suitable for trace analysis of photoactive substances in complex matrices.
- Chromatographic separation techniques with enhanced detection limits: High-performance liquid chromatography and gas chromatography methods are optimized for photoactive compound detection. These techniques combine efficient separation with sensitive detection systems to achieve lower detection limits. The methods often incorporate specialized detectors and sample preparation procedures to minimize interference and maximize sensitivity for photoactive analytes.
- Electrochemical detection approaches: Electrochemical methods provide alternative detection strategies for photoactive compounds based on their redox properties. These techniques offer advantages in terms of selectivity and can achieve very low detection limits through signal amplification methods. The approaches include voltammetric and amperometric detection systems specifically designed for photoactive analyte quantification.
- Mass spectrometry-based detection systems: Mass spectrometry techniques provide highly specific and sensitive detection of photoactive compounds through molecular identification. These methods can achieve extremely low detection limits by utilizing ionization techniques optimized for photoactive substances. The approaches often combine chromatographic separation with mass spectrometric detection for enhanced specificity and quantification accuracy.
- Sample preparation and matrix effect minimization: Specialized sample preparation techniques are developed to improve detection limits by reducing matrix interference and concentrating photoactive compounds. These methods include extraction procedures, cleanup steps, and preconcentration techniques specifically designed for photoactive analytes. The approaches focus on eliminating background interference while preserving analyte integrity to achieve optimal detection performance.
02 Chromatographic separation and detection systems
Chromatographic methods combined with sensitive detection systems provide effective approaches for determining detection limits of photoactive compounds. These systems separate complex mixtures and enable individual component analysis with high precision. The integration of advanced detection technologies with chromatographic separation enhances the ability to identify and quantify trace amounts of photoactive substances in various applications.Expand Specific Solutions03 Electrochemical detection approaches
Electrochemical methods offer sensitive detection capabilities for photoactive compounds by measuring electrical responses upon light activation or chemical interaction. These techniques can achieve low detection limits through optimized electrode configurations and measurement protocols. The electrochemical approach provides real-time monitoring capabilities and can be adapted for various photoactive compound types with different redox properties.Expand Specific Solutions04 Biosensor-based detection systems
Biological detection systems utilize living organisms or biological components to detect photoactive compounds with high specificity and sensitivity. These biosensors can achieve extremely low detection limits by leveraging natural biological recognition mechanisms and amplification processes. The biological approach offers advantages in terms of selectivity and can be designed for specific photoactive compound classes or environmental monitoring applications.Expand Specific Solutions05 Optical sensor technologies and photonic detection
Advanced optical sensor technologies employ photonic principles to detect photoactive compounds with enhanced sensitivity and reduced detection limits. These systems utilize specialized optical components, light sources, and detection mechanisms optimized for photoactive substance identification. The photonic approach enables non-invasive detection methods and can be integrated into portable or automated detection platforms for various analytical applications.Expand Specific Solutions
Key Players in Photoactive Bioimaging Industry
The bioimaging field with nanomolar detection limits represents a rapidly evolving market driven by increasing demand for precision diagnostics and personalized medicine. The industry is experiencing significant growth, with market expansion fueled by advances in molecular imaging technologies and rising healthcare investments globally. The competitive landscape spans established pharmaceutical giants like Novartis AG and specialized imaging companies such as Bracco Imaging SpA and GE Healthcare AS, alongside emerging biotechnology firms like AmberGen Inc. and VisEn Medical Inc. Technology maturity varies considerably across players, with traditional contrast agent manufacturers like Nihon Medi-Physics demonstrating established capabilities, while innovative companies such as Immunomedics Inc. and Curis Inc. are advancing next-generation photoactive compounds. Academic institutions including Washington University in St. Louis, Boston University, and Sorbonne Université contribute fundamental research breakthroughs. The sector shows promising consolidation potential as companies like Surmodics Inc. and specialized research organizations collaborate to bridge the gap between laboratory discoveries and clinical applications, positioning the market for substantial technological advancement.
Bracco Imaging SpA
Technical Solution: Bracco Imaging has developed advanced gadolinium-based contrast agents and molecular imaging probes for MRI and CT imaging applications. Their photoactive compounds include gadolinium chelates with enhanced relaxivity properties, achieving detection sensitivities in the nanomolar range for targeted molecular imaging. The company's MultiHance and ProHance contrast agents demonstrate superior pharmacokinetic profiles with improved tissue contrast enhancement. Their research focuses on developing targeted contrast agents that can bind to specific biomarkers, enabling precise detection of pathological tissues at very low concentrations through optimized molecular design and chelation chemistry.
Strengths: Established market leader in contrast agents with proven clinical safety profiles and regulatory approvals. Weaknesses: Limited to traditional contrast mechanisms, may face challenges with newer fluorescent imaging modalities.
GE Healthcare AS
Technical Solution: GE Healthcare has developed comprehensive molecular imaging solutions including fluorescent probes and radiotracers for PET, SPECT, and optical imaging systems. Their photoactive compounds portfolio includes near-infrared fluorescent dyes and targeted molecular probes capable of nanomolar detection limits. The company's imaging agents utilize advanced fluorophore chemistry combined with targeting moieties for specific tissue localization. Their research emphasizes developing theranostic agents that combine diagnostic imaging capabilities with therapeutic functions, particularly for oncology applications where precise tumor detection and monitoring are critical for treatment planning.
Strengths: Comprehensive imaging platform integration with strong clinical validation and global distribution network. Weaknesses: High development costs and complex regulatory pathways for new molecular imaging agents.
Core Innovations in Ultra-Sensitive Photoactive Compounds
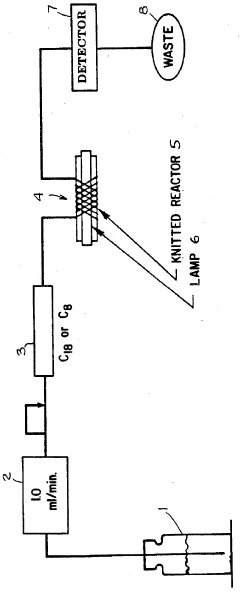
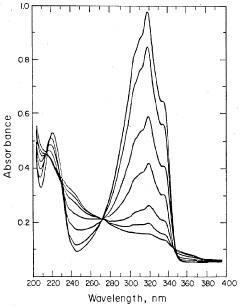
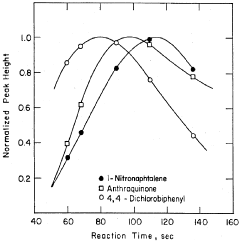
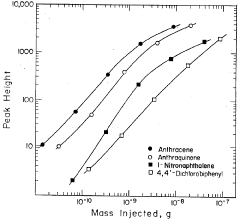
Method of improving the detection limits of UV-VIS absorbing compounds in HPLC by the use of a singlet oxygen trap
PatentInactiveUS4806485A
Innovation
- A post-column photochemical reaction method using a photochemical reactor with a singlet oxygen trap, such as substituted furans, to produce singlet oxygen, which reacts with analytes to enhance detectability without requiring oxygen removal, by promoting analyte molecules to an excited triplet state and transferring energy to oxygen in the mobile phase.
Photoactivatable compounds and uses thereof
PatentWO2023215497A1
Innovation
- Development of photoactivatable compounds with vinyl-extended-aryl azide moieties that undergo photoactivation to form reactive intermediates, enabling covalent linkages with biomolecules, and incorporating functional moieties for detection, enrichment, or bioactive properties.
Regulatory Framework for Photoactive Bioimaging Agents
The regulatory landscape for photoactive bioimaging agents designed to achieve nanomolar detection limits is governed by a complex framework of international and national guidelines. The Food and Drug Administration (FDA) in the United States classifies these agents under medical devices or drugs depending on their intended use, requiring comprehensive preclinical and clinical evaluation through the Investigational New Drug (IND) application process. Similarly, the European Medicines Agency (EMA) mandates adherence to the Clinical Trials Regulation for investigational medicinal products containing photoactive compounds.
Safety assessment protocols specifically address photosensitivity concerns, requiring extensive photobiological testing according to ISO 10993-17 standards. Regulatory bodies demand detailed characterization of absorption spectra, quantum yields, and phototoxicity profiles across relevant wavelength ranges. The International Council for Harmonisation (ICH) guidelines M3(R2) provide specific requirements for non-clinical safety studies, emphasizing the need for comprehensive genotoxicity and carcinogenicity assessments for compounds with light-activated properties.
Quality control standards for photoactive bioimaging agents follow Good Manufacturing Practice (GMP) guidelines with additional requirements for light-sensitive materials. The United States Pharmacopeia (USP) Chapter 1118 provides specific guidance for monitoring and controlling light exposure during manufacturing, storage, and distribution. Analytical method validation must demonstrate stability under various lighting conditions and establish appropriate photodegradation limits.
International harmonization efforts through the International Conference on Harmonisation have established unified standards for photosafety evaluation. The OECD Test Guideline 432 for in vitro 3T3 Neutral Red Uptake Phototoxicity Test serves as the primary screening method for identifying phototoxic potential. Regulatory submissions must include comprehensive photostability studies conducted according to ICH Q1B guidelines, demonstrating compound integrity under controlled light exposure conditions.
Post-market surveillance requirements mandate continuous monitoring of adverse events related to photosensitivity reactions. Regulatory frameworks increasingly emphasize risk management plans that address potential photobiological hazards and establish appropriate patient monitoring protocols for clinical applications involving high-sensitivity photoactive compounds.
Safety assessment protocols specifically address photosensitivity concerns, requiring extensive photobiological testing according to ISO 10993-17 standards. Regulatory bodies demand detailed characterization of absorption spectra, quantum yields, and phototoxicity profiles across relevant wavelength ranges. The International Council for Harmonisation (ICH) guidelines M3(R2) provide specific requirements for non-clinical safety studies, emphasizing the need for comprehensive genotoxicity and carcinogenicity assessments for compounds with light-activated properties.
Quality control standards for photoactive bioimaging agents follow Good Manufacturing Practice (GMP) guidelines with additional requirements for light-sensitive materials. The United States Pharmacopeia (USP) Chapter 1118 provides specific guidance for monitoring and controlling light exposure during manufacturing, storage, and distribution. Analytical method validation must demonstrate stability under various lighting conditions and establish appropriate photodegradation limits.
International harmonization efforts through the International Conference on Harmonisation have established unified standards for photosafety evaluation. The OECD Test Guideline 432 for in vitro 3T3 Neutral Red Uptake Phototoxicity Test serves as the primary screening method for identifying phototoxic potential. Regulatory submissions must include comprehensive photostability studies conducted according to ICH Q1B guidelines, demonstrating compound integrity under controlled light exposure conditions.
Post-market surveillance requirements mandate continuous monitoring of adverse events related to photosensitivity reactions. Regulatory frameworks increasingly emphasize risk management plans that address potential photobiological hazards and establish appropriate patient monitoring protocols for clinical applications involving high-sensitivity photoactive compounds.
Safety Assessment of Photoactive Compounds in Bioimaging
The safety assessment of photoactive compounds in bioimaging represents a critical evaluation framework that must be rigorously applied when selecting compounds capable of achieving nanomolar detection limits. This assessment encompasses multiple dimensions of biological safety, ranging from acute cytotoxicity to long-term biocompatibility, ensuring that the enhanced sensitivity does not compromise patient safety or experimental integrity.
Cytotoxicity evaluation forms the foundation of safety assessment, particularly for compounds designed to operate at nanomolar concentrations. While lower concentrations generally reduce toxicity risks, the enhanced photophysical properties required for nanomolar detection often involve structural modifications that may introduce unexpected biological interactions. Standard cytotoxicity assays, including MTT, XTT, and live/dead staining protocols, must be conducted across multiple cell lines to establish comprehensive toxicity profiles.
Phototoxicity assessment becomes increasingly critical as photoactive compounds with enhanced quantum yields and extended excitation wavelengths are developed for improved detection sensitivity. The combination of light exposure and compound presence can generate reactive oxygen species or other photochemical products that may cause cellular damage even at nanomolar concentrations. Standardized phototoxicity testing protocols, following guidelines such as those established by the International Conference on Harmonisation, provide essential safety data.
Biodistribution and clearance kinetics require careful evaluation, as compounds optimized for nanomolar detection may exhibit altered pharmacokinetic properties. Enhanced binding affinity or modified molecular structures designed to improve detection limits can potentially affect tissue distribution patterns, cellular uptake mechanisms, and elimination pathways. These factors directly impact both imaging quality and safety profiles.
Genotoxicity screening represents another essential component, particularly for compounds intended for repeated use or long-term studies. The structural modifications that enable nanomolar detection sensitivity may introduce DNA-binding capabilities or generate metabolites with mutagenic potential. Comprehensive genotoxicity testing, including Ames testing and chromosomal aberration assays, ensures genetic safety.
Regulatory compliance considerations must align with intended applications, whether for research use, preclinical studies, or clinical applications. Different regulatory frameworks apply varying safety standards, with clinical applications requiring the most stringent safety documentation. The enhanced detection capabilities of nanomolar-sensitive compounds must be balanced against regulatory requirements for comprehensive safety data packages.
Cytotoxicity evaluation forms the foundation of safety assessment, particularly for compounds designed to operate at nanomolar concentrations. While lower concentrations generally reduce toxicity risks, the enhanced photophysical properties required for nanomolar detection often involve structural modifications that may introduce unexpected biological interactions. Standard cytotoxicity assays, including MTT, XTT, and live/dead staining protocols, must be conducted across multiple cell lines to establish comprehensive toxicity profiles.
Phototoxicity assessment becomes increasingly critical as photoactive compounds with enhanced quantum yields and extended excitation wavelengths are developed for improved detection sensitivity. The combination of light exposure and compound presence can generate reactive oxygen species or other photochemical products that may cause cellular damage even at nanomolar concentrations. Standardized phototoxicity testing protocols, following guidelines such as those established by the International Conference on Harmonisation, provide essential safety data.
Biodistribution and clearance kinetics require careful evaluation, as compounds optimized for nanomolar detection may exhibit altered pharmacokinetic properties. Enhanced binding affinity or modified molecular structures designed to improve detection limits can potentially affect tissue distribution patterns, cellular uptake mechanisms, and elimination pathways. These factors directly impact both imaging quality and safety profiles.
Genotoxicity screening represents another essential component, particularly for compounds intended for repeated use or long-term studies. The structural modifications that enable nanomolar detection sensitivity may introduce DNA-binding capabilities or generate metabolites with mutagenic potential. Comprehensive genotoxicity testing, including Ames testing and chromosomal aberration assays, ensures genetic safety.
Regulatory compliance considerations must align with intended applications, whether for research use, preclinical studies, or clinical applications. Different regulatory frameworks apply varying safety standards, with clinical applications requiring the most stringent safety documentation. The enhanced detection capabilities of nanomolar-sensitive compounds must be balanced against regulatory requirements for comprehensive safety data packages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!