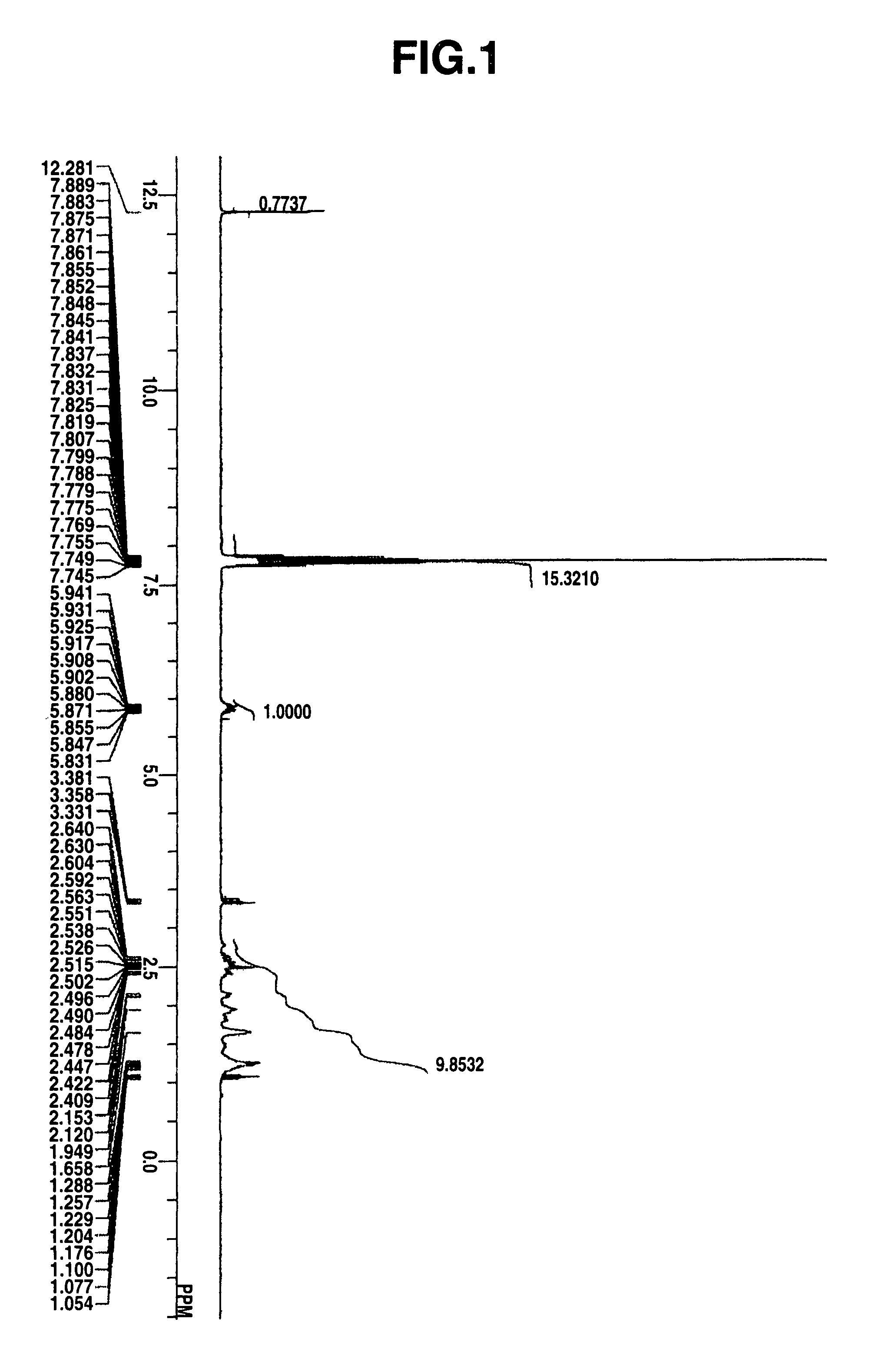
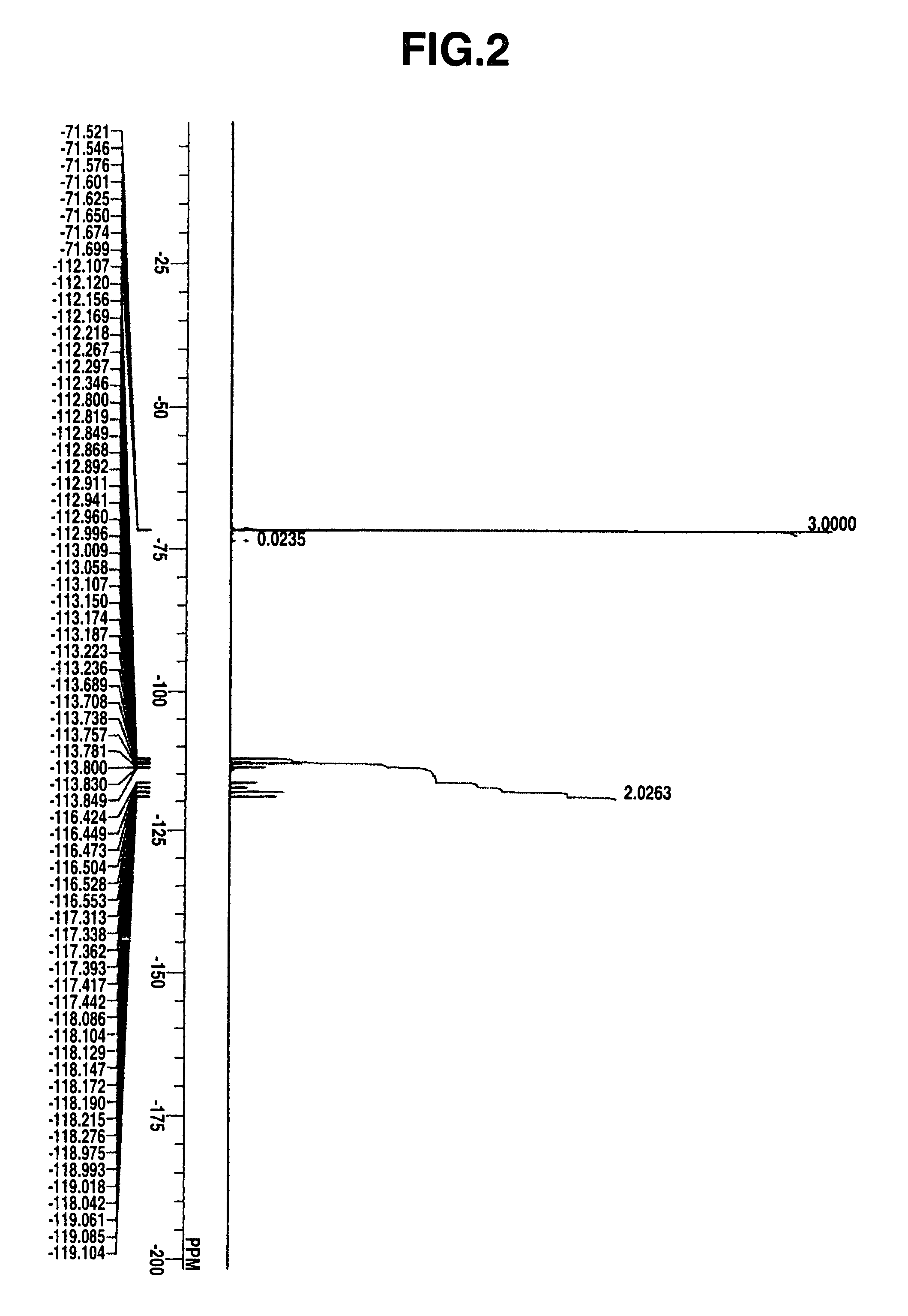
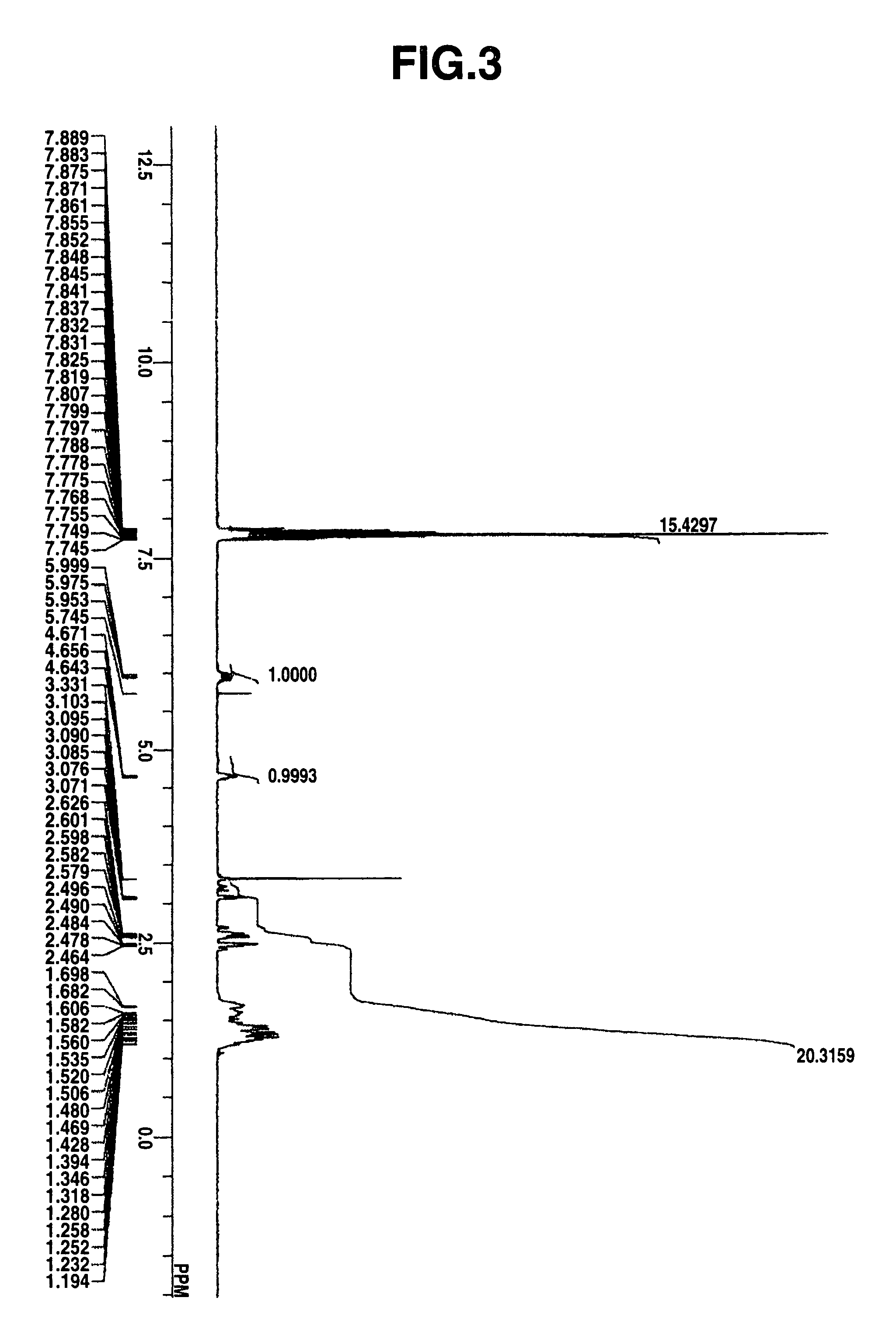
Select Photoactive Compound For Photoacid Generation Efficiency
DEC 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photoacid Generation Technology Background and Objectives
Photoacid generation technology represents a cornerstone of modern photolithography and photochemical processes, fundamentally enabling the precise control of chemical reactions through light-induced acid formation. This technology has evolved from simple photochemical systems to sophisticated molecular architectures capable of generating strong acids upon photon absorption, serving as catalysts for subsequent chemical transformations.
The historical development of photoacid generators (PAGs) traces back to the 1970s when the semiconductor industry demanded higher resolution patterning capabilities. Early systems relied on basic photoactive compounds that exhibited limited efficiency and selectivity. The evolution progressed through multiple generations, from onium salt-based systems to modern sulfonium and iodonium compounds, each iteration addressing specific limitations in quantum efficiency, thermal stability, and acid strength.
Contemporary photoacid generation systems operate on the principle of photoinduced electron transfer or direct photolysis, where specific wavelengths of light trigger molecular rearrangements leading to proton release. The efficiency of this process depends critically on the molecular structure of the photoactive compound, its absorption characteristics, and the quantum yield of acid formation. Modern applications span beyond traditional photolithography to include 3D printing, surface modification, and advanced materials processing.
The primary technical objective centers on optimizing the selection criteria for photoactive compounds to maximize photoacid generation efficiency. This involves understanding the relationship between molecular structure and photochemical performance, including factors such as absorption cross-section, excited state dynamics, and reaction pathway selectivity. Enhanced efficiency translates directly to improved process throughput, reduced exposure times, and better pattern fidelity in industrial applications.
Current research directions focus on developing next-generation photoactive compounds with tailored spectral responses, improved solubility characteristics, and enhanced thermal stability. The integration of computational chemistry approaches enables rational design of molecular structures optimized for specific wavelength ranges and application requirements. These advances aim to establish comprehensive selection methodologies that can predict and optimize photoacid generation performance across diverse operational conditions and substrate materials.
The historical development of photoacid generators (PAGs) traces back to the 1970s when the semiconductor industry demanded higher resolution patterning capabilities. Early systems relied on basic photoactive compounds that exhibited limited efficiency and selectivity. The evolution progressed through multiple generations, from onium salt-based systems to modern sulfonium and iodonium compounds, each iteration addressing specific limitations in quantum efficiency, thermal stability, and acid strength.
Contemporary photoacid generation systems operate on the principle of photoinduced electron transfer or direct photolysis, where specific wavelengths of light trigger molecular rearrangements leading to proton release. The efficiency of this process depends critically on the molecular structure of the photoactive compound, its absorption characteristics, and the quantum yield of acid formation. Modern applications span beyond traditional photolithography to include 3D printing, surface modification, and advanced materials processing.
The primary technical objective centers on optimizing the selection criteria for photoactive compounds to maximize photoacid generation efficiency. This involves understanding the relationship between molecular structure and photochemical performance, including factors such as absorption cross-section, excited state dynamics, and reaction pathway selectivity. Enhanced efficiency translates directly to improved process throughput, reduced exposure times, and better pattern fidelity in industrial applications.
Current research directions focus on developing next-generation photoactive compounds with tailored spectral responses, improved solubility characteristics, and enhanced thermal stability. The integration of computational chemistry approaches enables rational design of molecular structures optimized for specific wavelength ranges and application requirements. These advances aim to establish comprehensive selection methodologies that can predict and optimize photoacid generation performance across diverse operational conditions and substrate materials.
Market Demand for Advanced Photoacid Generator Applications
The semiconductor industry represents the largest and most critical market segment for advanced photoacid generators, driven by the continuous miniaturization of electronic devices and the transition to extreme ultraviolet lithography. As chip manufacturers push toward sub-3nm process nodes, the demand for highly efficient photoactive compounds has intensified significantly. The precision required for creating increasingly smaller circuit patterns necessitates photoacid generators with superior quantum efficiency and enhanced sensitivity to specific wavelengths.
Display technology manufacturing constitutes another substantial market driver, particularly in the production of high-resolution OLED and micro-LED displays. The growing consumer demand for smartphones, tablets, and large-format displays with enhanced pixel density requires photolithographic processes that can achieve exceptional pattern fidelity. Advanced photoacid generators enable manufacturers to produce the intricate thin-film transistor arrays essential for next-generation display technologies.
The printed circuit board industry has emerged as a significant consumer of specialized photoactive compounds, especially as 5G infrastructure deployment accelerates globally. High-frequency applications demand PCBs with tighter tolerances and more complex multilayer structures, driving the need for photoacid generators that can deliver consistent performance across varying substrate materials and processing conditions.
Emerging applications in flexible electronics and wearable devices are creating new market opportunities for photoacid generators optimized for non-traditional substrates. The ability to process photoresists on plastic and other flexible materials requires photoactive compounds with modified chemical properties and enhanced compatibility with low-temperature processing conditions.
The automotive electronics sector represents a rapidly expanding market segment, particularly with the proliferation of electric vehicles and autonomous driving systems. Advanced driver assistance systems and battery management electronics require highly reliable semiconductor components manufactured using state-of-the-art lithographic processes, further driving demand for efficient photoacid generation systems.
Biomedical device manufacturing has begun adopting advanced photolithographic techniques for producing microfluidic devices and biosensors, creating niche but high-value market opportunities. These applications often require biocompatible photoactive compounds with specialized performance characteristics tailored to medical device manufacturing requirements.
Display technology manufacturing constitutes another substantial market driver, particularly in the production of high-resolution OLED and micro-LED displays. The growing consumer demand for smartphones, tablets, and large-format displays with enhanced pixel density requires photolithographic processes that can achieve exceptional pattern fidelity. Advanced photoacid generators enable manufacturers to produce the intricate thin-film transistor arrays essential for next-generation display technologies.
The printed circuit board industry has emerged as a significant consumer of specialized photoactive compounds, especially as 5G infrastructure deployment accelerates globally. High-frequency applications demand PCBs with tighter tolerances and more complex multilayer structures, driving the need for photoacid generators that can deliver consistent performance across varying substrate materials and processing conditions.
Emerging applications in flexible electronics and wearable devices are creating new market opportunities for photoacid generators optimized for non-traditional substrates. The ability to process photoresists on plastic and other flexible materials requires photoactive compounds with modified chemical properties and enhanced compatibility with low-temperature processing conditions.
The automotive electronics sector represents a rapidly expanding market segment, particularly with the proliferation of electric vehicles and autonomous driving systems. Advanced driver assistance systems and battery management electronics require highly reliable semiconductor components manufactured using state-of-the-art lithographic processes, further driving demand for efficient photoacid generation systems.
Biomedical device manufacturing has begun adopting advanced photolithographic techniques for producing microfluidic devices and biosensors, creating niche but high-value market opportunities. These applications often require biocompatible photoactive compounds with specialized performance characteristics tailored to medical device manufacturing requirements.
Current Status and Challenges in Photoactive Compound Selection
The selection of photoactive compounds for photoacid generation represents a critical challenge in modern photolithography and photopolymerization applications. Current photoacid generators (PAGs) primarily rely on onium salts, particularly iodonium and sulfonium compounds, which have dominated the market for decades. These compounds demonstrate reliable acid generation upon UV or deep-UV exposure, but their efficiency remains limited by fundamental photophysical constraints and molecular design limitations.
Contemporary PAG systems face significant efficiency bottlenecks stemming from competing photochemical pathways that reduce quantum yields. Many existing photoactive compounds suffer from incomplete photolysis, where only a fraction of absorbed photons result in productive acid generation. This inefficiency becomes particularly problematic in advanced semiconductor manufacturing, where precise control over acid concentration gradients is essential for achieving sub-10nm feature resolution.
The spectral matching challenge represents another major obstacle in current photoactive compound selection. Most established PAGs exhibit absorption maxima that poorly align with commonly used light sources, particularly in extreme ultraviolet (EUV) lithography at 13.5nm wavelength. This mismatch necessitates the use of photosensitizers or higher exposure doses, introducing additional complexity and potential side reactions that compromise pattern fidelity.
Thermal stability issues plague many high-efficiency photoactive candidates, limiting their practical application in industrial processes. Compounds that demonstrate superior photoacid generation often exhibit premature thermal decomposition during storage or processing, leading to background fogging and reduced shelf life. This trade-off between photochemical efficiency and thermal stability constrains the available design space for new PAG development.
Solubility and compatibility challenges further complicate photoactive compound selection, particularly in advanced resist formulations. Many promising PAG candidates exhibit poor solubility in environmentally friendly solvents or demonstrate incompatibility with specific polymer matrices, limiting their integration into commercial photoresist systems. The push toward green chemistry in semiconductor manufacturing has intensified these compatibility requirements.
Current analytical methods for evaluating photoacid generation efficiency also present limitations, often failing to capture the complex interplay between photochemical quantum yield, acid diffusion characteristics, and resist performance. This measurement gap hinders the systematic optimization of photoactive compound selection and slows the development of next-generation PAG systems with enhanced efficiency profiles.
Contemporary PAG systems face significant efficiency bottlenecks stemming from competing photochemical pathways that reduce quantum yields. Many existing photoactive compounds suffer from incomplete photolysis, where only a fraction of absorbed photons result in productive acid generation. This inefficiency becomes particularly problematic in advanced semiconductor manufacturing, where precise control over acid concentration gradients is essential for achieving sub-10nm feature resolution.
The spectral matching challenge represents another major obstacle in current photoactive compound selection. Most established PAGs exhibit absorption maxima that poorly align with commonly used light sources, particularly in extreme ultraviolet (EUV) lithography at 13.5nm wavelength. This mismatch necessitates the use of photosensitizers or higher exposure doses, introducing additional complexity and potential side reactions that compromise pattern fidelity.
Thermal stability issues plague many high-efficiency photoactive candidates, limiting their practical application in industrial processes. Compounds that demonstrate superior photoacid generation often exhibit premature thermal decomposition during storage or processing, leading to background fogging and reduced shelf life. This trade-off between photochemical efficiency and thermal stability constrains the available design space for new PAG development.
Solubility and compatibility challenges further complicate photoactive compound selection, particularly in advanced resist formulations. Many promising PAG candidates exhibit poor solubility in environmentally friendly solvents or demonstrate incompatibility with specific polymer matrices, limiting their integration into commercial photoresist systems. The push toward green chemistry in semiconductor manufacturing has intensified these compatibility requirements.
Current analytical methods for evaluating photoacid generation efficiency also present limitations, often failing to capture the complex interplay between photochemical quantum yield, acid diffusion characteristics, and resist performance. This measurement gap hinders the systematic optimization of photoactive compound selection and slows the development of next-generation PAG systems with enhanced efficiency profiles.
Current Photoactive Compound Selection Methodologies
01 Photoacid generator compounds with enhanced quantum efficiency
Development of novel photoacid generator compounds that exhibit improved quantum efficiency for acid generation upon exposure to light. These compounds are designed with specific molecular structures that optimize the photochemical conversion process, resulting in higher acid generation rates per photon absorbed. The enhanced efficiency is achieved through molecular engineering approaches that minimize energy loss pathways and maximize productive photochemical reactions.- Photoacid generator molecular structure optimization: The molecular structure of photoacid generators can be optimized to enhance photoacid generation efficiency. This involves modifying the chemical backbone, substituent groups, and chromophore systems to improve light absorption and acid release properties. Structural modifications can include incorporating electron-withdrawing groups, extending conjugation systems, and optimizing the leaving group characteristics to achieve higher quantum yields for acid generation.
- Wavelength-specific photoacid generation systems: Development of photoacid generators that are specifically designed to operate efficiently at particular wavelengths or wavelength ranges. These systems are tailored to match specific light sources and applications, with enhanced absorption coefficients and photochemical conversion rates at target wavelengths. The optimization includes tuning the electronic transitions and excited state properties to maximize photoacid generation under specific irradiation conditions.
- Sensitizer-enhanced photoacid generation: Incorporation of photosensitizers or sensitizing compounds to improve the efficiency of photoacid generation through energy transfer mechanisms. These systems utilize sensitizers that can absorb light more effectively and transfer the energy to the photoacid generator, resulting in enhanced acid generation rates. The approach allows for improved performance under low-intensity illumination or at wavelengths where the photoacid generator has poor direct absorption.
- Multi-component photoacid generation systems: Development of multi-component formulations that combine different photoacid generators or incorporate additional components to achieve synergistic effects in acid generation efficiency. These systems may include combinations of different photoacid generator types, co-initiators, or auxiliary compounds that work together to enhance overall photochemical performance. The multi-component approach allows for broader spectral response and improved quantum efficiency.
- Environmental and matrix effects on photoacid generation: Investigation and optimization of how environmental factors and matrix compositions affect photoacid generation efficiency. This includes studying the influence of solvent systems, polymer matrices, temperature, oxygen presence, and other environmental parameters on the photochemical processes. Understanding these effects allows for the design of optimized formulations and processing conditions that maximize acid generation efficiency in specific application environments.
02 Optimization of photoacid generator molecular structure
Systematic modification of photoacid generator molecular architectures to improve photoacid generation efficiency. This involves designing compounds with specific chromophore systems, electron-withdrawing groups, and leaving group arrangements that facilitate efficient photolysis and subsequent acid release. The structural optimization focuses on enhancing light absorption characteristics and promoting favorable photochemical pathways for acid generation.Expand Specific Solutions03 Sensitizer systems for enhanced photoacid generation
Implementation of sensitizer compounds that work in conjunction with photoacid generators to improve overall acid generation efficiency. These systems utilize energy transfer mechanisms or electron transfer processes to enhance the photochemical activation of acid generators. The sensitizers can extend the effective wavelength range and increase the overall quantum yield of the photoacid generation process.Expand Specific Solutions04 Matrix and formulation effects on photoacid generation efficiency
Investigation of how the surrounding matrix and formulation components influence the efficiency of photoacid generation. This includes the effects of polymer hosts, solvents, additives, and other formulation ingredients on the photochemical behavior of acid generators. The optimization involves controlling factors such as molecular mobility, local environment polarity, and intermolecular interactions that can affect the photoacid generation process.Expand Specific Solutions05 Measurement and characterization methods for photoacid generation efficiency
Development of analytical techniques and methodologies for accurately measuring and characterizing the efficiency of photoacid generation processes. These methods include spectroscopic approaches, chemical indicators, and quantitative analysis techniques that can determine quantum yields, reaction kinetics, and overall performance metrics of photoacid generators under various conditions.Expand Specific Solutions
Key Players in Photoacid Generator and Photoactive Materials Industry
The photoacid generation efficiency technology sector represents a mature, specialized market within the broader semiconductor and electronic materials industry. The market demonstrates steady growth driven by increasing demand for advanced lithography processes in semiconductor manufacturing and display technologies. Key players exhibit varying levels of technological sophistication, with established Japanese companies like Tokyo Ohka Kogyo, JSR Corp., and Shin-Etsu Chemical leading in photoresist and photoacid generator development. Sumitomo Chemical and San-Apro demonstrate specialized expertise in acid catalyst systems, while multinational corporations such as DuPont Electronic Materials and FUJIFILM Electronic Materials leverage extensive R&D capabilities. The competitive landscape shows high technical barriers to entry, with companies like Samsung Electronics and SK Hynix representing major end-users driving innovation requirements. Technology maturity varies across applications, with semiconductor photolithography showing advanced development while emerging applications in specialty coatings and 3D printing remain in earlier stages.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a comprehensive portfolio of photoactive compounds for photoacid generation, focusing on triarylsulfonium and diaryliodonium salts with optimized counteranions. Their PAG technology emphasizes molecular engineering to achieve high photoacid generation efficiency while maintaining excellent solubility in organic solvents and photoresist formulations. The company's approach involves systematic structure-activity relationship studies to identify optimal chromophore configurations that maximize light absorption and subsequent acid generation. Their PAGs incorporate bulky organic anions that provide controlled acid strength and diffusion properties, enabling precise pattern formation in advanced lithography processes. Recent developments include PAGs with enhanced transparency at deep UV wavelengths and improved thermal stability for high-temperature processing applications.
Strengths: Strong chemical manufacturing capabilities, extensive experience in specialty chemicals, robust supply chain infrastructure. Weaknesses: Intense competition in the PAG market, pressure from cost-sensitive customers in semiconductor industry.
TOKYO OHKA KOGYO CO., LTD.
Technical Solution: Tokyo Ohka Kogyo has developed advanced photoacid generator (PAG) compounds specifically optimized for extreme ultraviolet (EUV) lithography applications. Their proprietary sulfonium and iodonium salt-based PAGs demonstrate enhanced photoacid generation efficiency through improved quantum yield mechanisms. The company's PAG formulations incorporate novel chromophore structures that maximize photon absorption at 193nm and 13.5nm wavelengths, achieving acid generation quantum yields exceeding 0.8. Their molecular design approach focuses on optimizing the photolysis pathway to minimize side reactions while maximizing proton release efficiency. The PAGs are engineered with tailored solubility parameters and thermal stability characteristics to ensure uniform distribution in photoresist matrices and maintain performance under processing conditions.
Strengths: Industry-leading expertise in photoresist chemistry with decades of experience, strong patent portfolio in PAG technology. Weaknesses: Limited diversification outside traditional lithography markets, high R&D costs for next-generation materials.
Core Innovations in High-Efficiency Photoacid Generation
Photoacid generator
PatentActiveUS20240069442A1
Innovation
- A novel photoacid generator compound cation with high absorption cross-section for EUV photons, selected from elements like In, Sn, Sb, Tl, Pb, or Bi, which upon EUV exposure generates intermediate radicals and releases a proton to form a Brønsted acid, increasing sensitivity and reducing toxicity and waste.
Photoacid generators, resist compositions, and patterning process
PatentActiveUS7527912B2
Innovation
- Development of novel photoacid generators that generate sulfonic acids with specific structures, such as sulfonium, iodonium, and oxime sulfonate compounds, which are designed to minimize water dissolution, control acid diffusion, and be degradable, using 1,1,1,3,3,3-hexafluoro-2-propanol derivatives with carbonyl and carboxyl groups for improved compatibility and environmental safety.
Environmental Impact Assessment of Photoacid Generators
The environmental implications of photoacid generators (PAGs) in semiconductor manufacturing and photolithography applications present multifaceted challenges that require comprehensive assessment across their entire lifecycle. These photoactive compounds, while essential for high-resolution pattern formation, introduce various environmental concerns ranging from manufacturing emissions to end-of-life disposal considerations.
During the production phase, PAG synthesis typically involves halogenated organic compounds and heavy metal catalysts, generating volatile organic compounds (VOCs) and potentially hazardous byproducts. Manufacturing facilities must implement stringent emission controls to minimize atmospheric releases of these compounds, which can contribute to air quality degradation and pose occupational health risks. The energy-intensive synthesis processes also contribute to the overall carbon footprint of semiconductor manufacturing operations.
Workplace exposure represents a critical environmental health concern, as many PAGs exhibit properties that may cause respiratory irritation, skin sensitization, or other adverse health effects. Onium salts, commonly used as PAGs, can release acidic species upon photolysis, creating corrosive environments that require specialized handling protocols and waste management systems. The implementation of closed-loop processing systems and advanced ventilation technologies becomes essential to minimize worker exposure and environmental releases.
Aquatic ecosystem impact assessment reveals particular concerns regarding PAG persistence and bioaccumulation potential. Many photoactive compounds demonstrate limited biodegradability, leading to accumulation in wastewater treatment systems and potential discharge into water bodies. The acidic photolysis products can alter local pH conditions, affecting aquatic organisms and disrupting natural ecosystem balance.
Waste stream management poses significant challenges due to the complex chemical nature of spent PAG solutions and contaminated substrates. Traditional incineration methods may generate toxic combustion products, while landfill disposal risks groundwater contamination through leachate formation. Advanced treatment technologies, including photocatalytic degradation and specialized chemical neutralization processes, are increasingly necessary to ensure safe disposal.
Regulatory compliance frameworks continue evolving to address emerging environmental concerns associated with PAG usage. Recent assessments indicate growing scrutiny regarding perfluorinated PAG compounds, which exhibit exceptional persistence and potential for long-range environmental transport. This regulatory landscape drives innovation toward more environmentally benign alternatives while maintaining the high performance standards required for advanced lithographic applications.
During the production phase, PAG synthesis typically involves halogenated organic compounds and heavy metal catalysts, generating volatile organic compounds (VOCs) and potentially hazardous byproducts. Manufacturing facilities must implement stringent emission controls to minimize atmospheric releases of these compounds, which can contribute to air quality degradation and pose occupational health risks. The energy-intensive synthesis processes also contribute to the overall carbon footprint of semiconductor manufacturing operations.
Workplace exposure represents a critical environmental health concern, as many PAGs exhibit properties that may cause respiratory irritation, skin sensitization, or other adverse health effects. Onium salts, commonly used as PAGs, can release acidic species upon photolysis, creating corrosive environments that require specialized handling protocols and waste management systems. The implementation of closed-loop processing systems and advanced ventilation technologies becomes essential to minimize worker exposure and environmental releases.
Aquatic ecosystem impact assessment reveals particular concerns regarding PAG persistence and bioaccumulation potential. Many photoactive compounds demonstrate limited biodegradability, leading to accumulation in wastewater treatment systems and potential discharge into water bodies. The acidic photolysis products can alter local pH conditions, affecting aquatic organisms and disrupting natural ecosystem balance.
Waste stream management poses significant challenges due to the complex chemical nature of spent PAG solutions and contaminated substrates. Traditional incineration methods may generate toxic combustion products, while landfill disposal risks groundwater contamination through leachate formation. Advanced treatment technologies, including photocatalytic degradation and specialized chemical neutralization processes, are increasingly necessary to ensure safe disposal.
Regulatory compliance frameworks continue evolving to address emerging environmental concerns associated with PAG usage. Recent assessments indicate growing scrutiny regarding perfluorinated PAG compounds, which exhibit exceptional persistence and potential for long-range environmental transport. This regulatory landscape drives innovation toward more environmentally benign alternatives while maintaining the high performance standards required for advanced lithographic applications.
Safety Protocols for Photoactive Compound Handling
The handling of photoactive compounds for photoacid generation requires comprehensive safety protocols due to their inherent photosensitivity and potential chemical reactivity. These compounds, including diazonium salts, onium salts, and halogenated organic molecules, present unique hazards that demand specialized protective measures beyond conventional chemical handling procedures.
Personal protective equipment forms the foundation of safe photoactive compound handling. Workers must utilize UV-blocking safety glasses or face shields to prevent accidental photochemical activation through ambient light exposure. Chemical-resistant gloves made from nitrile or fluoropolymer materials are essential, as many photoactive compounds can penetrate standard latex gloves. Full-coverage laboratory coats with long sleeves provide additional skin protection, while closed-toe shoes prevent accidental contact with spilled materials.
Environmental controls play a critical role in maintaining safe working conditions. Laboratory spaces must be equipped with amber or red lighting systems that minimize UV and blue light exposure, which can trigger unwanted photochemical reactions. Ventilation systems should maintain negative pressure with minimum air exchange rates of 6-12 changes per hour to prevent accumulation of potentially toxic vapors or decomposition products.
Storage protocols require specialized considerations for photoactive compounds. Materials must be stored in opaque, light-tight containers made from chemically compatible materials such as amber glass or UV-blocking polymers. Temperature control is crucial, with most photoactive compounds requiring storage between 2-8°C to maintain stability and prevent thermal decomposition. Segregation from incompatible materials, particularly strong bases, reducing agents, and other photosensitive chemicals, prevents dangerous cross-reactions.
Emergency response procedures must address both chemical exposure and photochemical activation scenarios. Immediate containment protocols include covering spilled materials with opaque barriers to prevent light-induced reactions while implementing standard chemical spill cleanup procedures. Emergency eyewash stations and safety showers should be readily accessible, with specific decontamination procedures established for different compound classes. Personnel training must encompass recognition of photochemical reaction symptoms and appropriate first aid measures for both skin and respiratory exposure incidents.
Personal protective equipment forms the foundation of safe photoactive compound handling. Workers must utilize UV-blocking safety glasses or face shields to prevent accidental photochemical activation through ambient light exposure. Chemical-resistant gloves made from nitrile or fluoropolymer materials are essential, as many photoactive compounds can penetrate standard latex gloves. Full-coverage laboratory coats with long sleeves provide additional skin protection, while closed-toe shoes prevent accidental contact with spilled materials.
Environmental controls play a critical role in maintaining safe working conditions. Laboratory spaces must be equipped with amber or red lighting systems that minimize UV and blue light exposure, which can trigger unwanted photochemical reactions. Ventilation systems should maintain negative pressure with minimum air exchange rates of 6-12 changes per hour to prevent accumulation of potentially toxic vapors or decomposition products.
Storage protocols require specialized considerations for photoactive compounds. Materials must be stored in opaque, light-tight containers made from chemically compatible materials such as amber glass or UV-blocking polymers. Temperature control is crucial, with most photoactive compounds requiring storage between 2-8°C to maintain stability and prevent thermal decomposition. Segregation from incompatible materials, particularly strong bases, reducing agents, and other photosensitive chemicals, prevents dangerous cross-reactions.
Emergency response procedures must address both chemical exposure and photochemical activation scenarios. Immediate containment protocols include covering spilled materials with opaque barriers to prevent light-induced reactions while implementing standard chemical spill cleanup procedures. Emergency eyewash stations and safety showers should be readily accessible, with specific decontamination procedures established for different compound classes. Personnel training must encompass recognition of photochemical reaction symptoms and appropriate first aid measures for both skin and respiratory exposure incidents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!