Ethyl propanoate, an ester derived from ethanol and propionic acid, is known for its fruity aroma and solvent capabilities. Beyond its sensory profile, it is gaining traction across green chemistry, biomedicine, and material engineering due to its volatility, reactivity, and low toxicity.
As industries strive for biodegradable alternatives, non-toxic synthesis platforms, and smart performance materials, ethyl propanoate offers versatility as a solvent, carrier, and building block in sustainable innovation.
This article delves into its material properties, domain-specific applications, comparative strengths and drawbacks, and the evolving research outlook—powered by insights from PatSnap’s Eureka AI Agent.
Material Composition & Key Properties
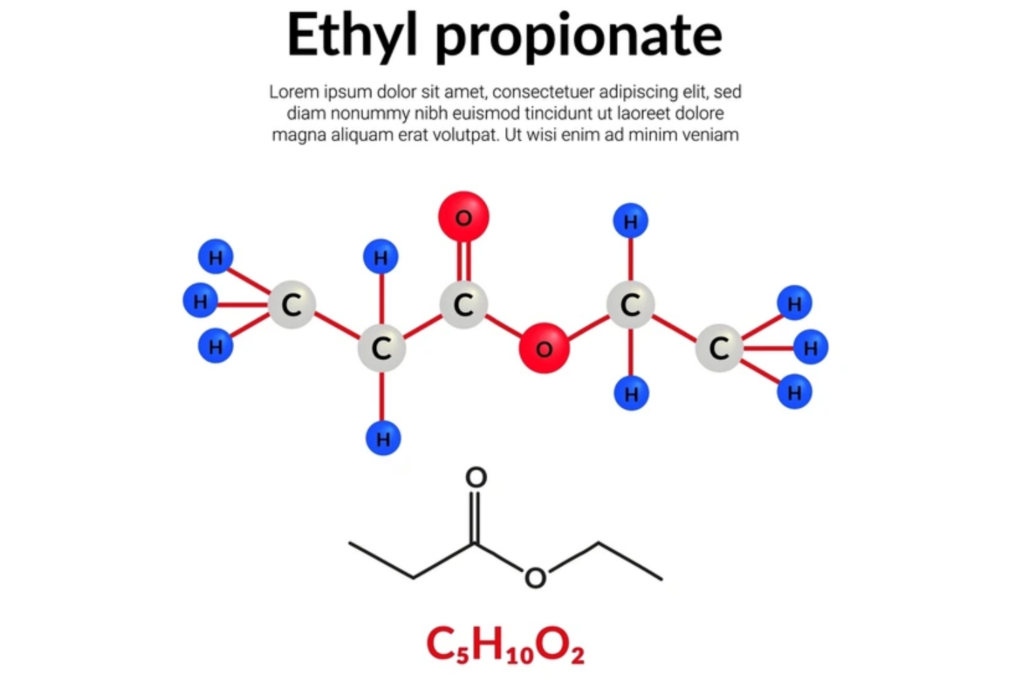
Ethyl propanoate (C5H10O2) is a short-chain ester with the following key attributes:
- Chemical Structure: CH3CH2COOCH2CH3
- Molecular Weight: 102.13 g/mol
- Boiling Point: ~99°C
- Solubility: Slightly soluble in water, fully soluble in alcohol and ether
- Key Functional Groups: Ester group (COO)
Typical Preparation:
- Esterification of ethanol with propionic acid using acid catalysts (e.g., H2SO4)
- Enzyme-catalyzed esterification for green synthesis
Performance Summary:
- ✓ Low toxicity and biodegradability
- ✓ Effective carrier in liquid formulations
- ✓ Evaporates cleanly, minimal residue
- ⚠ Not ideal for high-temperature or moisture-sensitive systems
Comparative Advantages & Limitations
✅ Advantages
- Eco-Friendly Solvent Profile
Ethyl propanoate is biodegradable, exhibits low aquatic toxicity, and complies with green chemistry principles—making it suitable for sustainable formulation in flavors, pharmaceuticals, and agrochemicals. - Pleasant Odor & Volatility
Its fruity aroma and appropriate vapor pressure make it ideal for use in food flavorings, perfumes, and aerosol systems where volatility and olfactory profiles are essential. - Synthetic Versatility
The ester group is reactive yet stable under mild conditions, enabling its use as an intermediate in various organic syntheses, including pharmaceuticals and fine chemicals. - Low Viscosity & Surface Tension
These physical properties contribute to its efficiency in coatings, inks, and polymer dispersions by improving flow, leveling, and penetration. - Regulatory Acceptance
Approved by bodies like the FDA and EFSA for use as a flavoring agent, reducing the regulatory burden in food and beverage applications.
⚠️ Limitations
- Limited Thermal Stability
Ethyl propanoate decomposes under high-temperature processing (>150°C), limiting its suitability in high-heat industrial applications such as some polymer curing or advanced manufacturing. - Flammability Risks
Its low flash point and high vapor pressure introduce handling and storage challenges, requiring strict compliance with flammable solvent protocols. - Odor Retention Issues in Polymers
While its fragrance is advantageous in some applications, ethyl propanoate may undesirably leach out or interact with host polymer matrices, affecting long-term stability or user experience. - Hydrolytic Sensitivity
In aqueous or humid conditions, especially at elevated temperatures, the ester bond may hydrolyze—posing challenges for use in waterborne systems unless properly stabilized. - High Cost Compared to Common Esters
For bulk solvent applications, ethyl propanoate may be less economically attractive than simpler esters like ethyl acetate or butyl acetate.
Application Domains
Energy Storage & Conversion
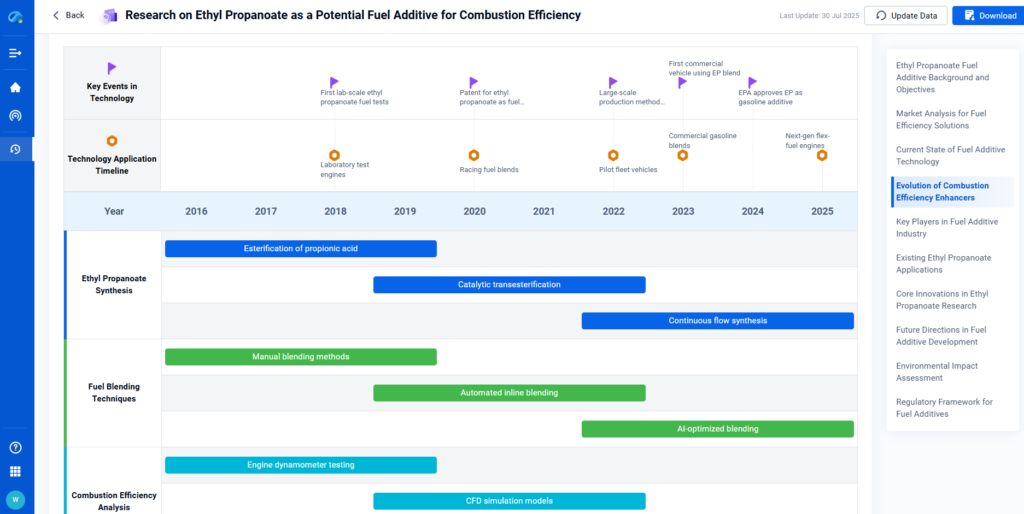
Modern energy systems require lightweight, stable, and efficient materials for fuel and battery applications. Ethyl Propanoate has emerged as a promising candidate due to its role in transesterification reactions and its potential as a fuel additive.
Ethyl Propanoate supports biodiesel production via transesterification, improves low-temperature fluidity in fuels, and contributes to electrocatalytic reactions relevant for energy generation.
Research Frontlines: Ethyl Propanoate is being studied for roles in lithium-ion battery electrodes, organic solar cell enhancement, and as a sustainable combustion enhancer.
Related Reports:
- Role of Ethyl Propanoate in Biodiesel Production
- How Ethyl Propanoate Enhances Biodiesel Flow Properties
- Ethyl Propanoate: Electrocatalytic Reduction for Chemical Synthesis
- Ethyl Propanoate as a Potential Fuel Additive for Combustion Efficiency
Organic Semiconductors & Photonic Materials
Ethyl Propanoate is gaining interest as a processing aid and solvent modifier in organic semiconducting materials, particularly in organic field-effect transistors (OFETs) and organic photovoltaics (OPVs). Its moderate polarity and volatility enable precise control over film morphology and molecular ordering, thereby impacting charge transport behavior and device efficiency. In next-generation OLEDs, it assists in fine-tuning layer deposition and crystallization kinetics, contributing to enhanced luminance and device longevity.
Nanotechnology & Smart Drug Delivery
In nanomedicine, Ethyl Propanoate serves as a green, biodegradable solvent for synthesizing lipid-based nanocarriers and polymeric micelles. Its low toxicity and favorable partition coefficient allow it to aid in the formation of nanoemulsions and nanoprecipitation processes used for controlled drug release. Research is increasingly exploring its role in stimuli-responsive nanocarriers and photothermal drug delivery systems where precise solvent compatibility is essential.
Advanced Polymer Systems
As a reactive diluent and co-solvent, Ethyl Propanoate modifies the thermal and mechanical properties of polymer films and thermoset resins. It can improve flexibility, processability, and solvent resistance in systems such as polyurethane dispersions and polyester-acrylate blends. In photocurable systems and resin-based 3D printing, it acts as a viscosity reducer while maintaining polymer network integrity during UV curing.
Aerospace & Propulsion Systems
In propulsion chemistry, Ethyl Propanoate functions as a combustion modifier and ignition delay regulator in hypergolic fuel blends. Its chemical structure enables controlled exothermic reactions with oxidizers like nitric acid derivatives. In satellite and spacecraft propulsion, it may contribute to hybrid propellant formulations aiming for higher impulse with lower environmental toxicity compared to traditional fuels.
High-Performance Lubricants & Brake Fluids
Its ester functionality and oxidative stability make Ethyl Propanoate a candidate additive in synthetic lubricants and brake fluids. It enhances viscosity index and cold-flow performance, which is vital in automotive and aerospace systems operating under extreme conditions. Additionally, its role in reducing friction and wear in boundary-lubrication regimes is actively studied through tribological testing and rheological simulations.
Specialty Coatings & Functional Surfaces
Ethyl Propanoate is utilized in solvent-borne coating formulations for high-durability applications such as flooring, elastomeric foams, and flexible packaging. It promotes uniform film formation, reduces drying time, and interacts favorably with plasticizers and crosslinking agents. Moreover, in photolithographic patterning, it acts as a developer component to refine resolution and contrast in micro- and nanoscale pattern transfers.
Future Outlook & Research Frontiers
As green chemistry matures, ethyl propanoate is poised to serve as both a model molecule and a scalable intermediate.
Emerging Directions:
- Bio-derived synthesis pathways from waste ethanol/propionic acid
- Use in smart drug delivery and transient electronics
- Expansion into bio-based lubricants and flavor encapsulants
- Sensor integration: Volatile fingerprinting in freshness diagnostics
Industry Challenges:
- Scaling enzymatic production
- Shelf-life tuning for packaging applications
- Regulatory alignment for food/pharma uses
Conclusion & Key Takeaways
Ethyl propanoate’s rise from a flavoring agent to a platform molecule underscores the power of small esters in sustainable chemistry. Its low toxicity, degradability, and molecular tunability make it valuable across combustion research, biomedicine, and eco-friendly materials.
Whether you’re an R&D scientist in green fuels or a product engineer in biodegradable packaging, EP opens new paths for scalable innovation.
Accelerate Innovation with PatSnap Eureka
Ready to unlock the full potential of ethyl propanoate?
PatSnap Eureka AI Agent empowers your team to:
- Identify white space opportunities
- Analyze chemical innovation landscapes
- Compare patents and track competitor insights in real-time
👉 Request a demo and start exploring today on Eureka.