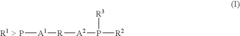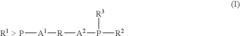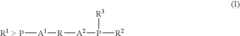Ethyl Propanoate: Electrocatalytic Reduction for Chemical Synthesis
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic Reduction Background and Objectives
Electrocatalytic reduction has emerged as a promising approach in the field of chemical synthesis, offering a sustainable and efficient alternative to traditional methods. This technology has gained significant attention in recent years due to its potential to address environmental concerns and improve process efficiency in the production of various chemicals, including ethyl propanoate.
The development of electrocatalytic reduction can be traced back to the early 20th century, with pioneering work in electrochemistry laying the foundation for modern applications. Over the decades, advancements in catalyst design, electrode materials, and electrochemical cell configurations have propelled this field forward, enabling more selective and energy-efficient transformations.
In the context of ethyl propanoate synthesis, electrocatalytic reduction presents an innovative approach to overcome the limitations of conventional production methods. Traditionally, ethyl propanoate has been synthesized through esterification reactions involving ethanol and propionic acid or its derivatives. These processes often require high temperatures, strong acids as catalysts, and generate significant amounts of waste.
The primary objective of employing electrocatalytic reduction for ethyl propanoate synthesis is to develop a more sustainable and atom-efficient production route. By harnessing electrical energy to drive the reduction of suitable precursors, this approach aims to minimize the use of harsh reagents, reduce energy consumption, and decrease the environmental footprint of the production process.
Key technical goals in this area include the design of highly selective and stable electrocatalysts capable of facilitating the desired reduction reaction while suppressing unwanted side reactions. Additionally, researchers are focused on optimizing reaction conditions, such as electrode potentials, electrolyte composition, and cell design, to enhance conversion rates and product yields.
Another critical objective is to scale up the electrocatalytic reduction process for industrial applications. This involves addressing challenges related to electrode stability, mass transfer limitations, and overall system efficiency. Researchers are exploring various reactor designs and continuous-flow systems to improve the practicality and economic viability of large-scale ethyl propanoate production via electrocatalytic reduction.
Furthermore, the development of this technology aligns with broader trends in the chemical industry towards greener and more sustainable manufacturing processes. As such, the research in this field also aims to contribute to the broader knowledge base of electrocatalytic transformations, potentially enabling the synthesis of a wide range of value-added chemicals and fuels through similar approaches.
The development of electrocatalytic reduction can be traced back to the early 20th century, with pioneering work in electrochemistry laying the foundation for modern applications. Over the decades, advancements in catalyst design, electrode materials, and electrochemical cell configurations have propelled this field forward, enabling more selective and energy-efficient transformations.
In the context of ethyl propanoate synthesis, electrocatalytic reduction presents an innovative approach to overcome the limitations of conventional production methods. Traditionally, ethyl propanoate has been synthesized through esterification reactions involving ethanol and propionic acid or its derivatives. These processes often require high temperatures, strong acids as catalysts, and generate significant amounts of waste.
The primary objective of employing electrocatalytic reduction for ethyl propanoate synthesis is to develop a more sustainable and atom-efficient production route. By harnessing electrical energy to drive the reduction of suitable precursors, this approach aims to minimize the use of harsh reagents, reduce energy consumption, and decrease the environmental footprint of the production process.
Key technical goals in this area include the design of highly selective and stable electrocatalysts capable of facilitating the desired reduction reaction while suppressing unwanted side reactions. Additionally, researchers are focused on optimizing reaction conditions, such as electrode potentials, electrolyte composition, and cell design, to enhance conversion rates and product yields.
Another critical objective is to scale up the electrocatalytic reduction process for industrial applications. This involves addressing challenges related to electrode stability, mass transfer limitations, and overall system efficiency. Researchers are exploring various reactor designs and continuous-flow systems to improve the practicality and economic viability of large-scale ethyl propanoate production via electrocatalytic reduction.
Furthermore, the development of this technology aligns with broader trends in the chemical industry towards greener and more sustainable manufacturing processes. As such, the research in this field also aims to contribute to the broader knowledge base of electrocatalytic transformations, potentially enabling the synthesis of a wide range of value-added chemicals and fuels through similar approaches.
Market Analysis for Ethyl Propanoate Synthesis
The market for ethyl propanoate synthesis through electrocatalytic reduction is experiencing significant growth, driven by increasing demand in various industries. This compound, also known as ethyl propionate, finds widespread applications in flavors and fragrances, solvents, and as an intermediate in pharmaceutical and agrochemical production.
In the flavor and fragrance industry, ethyl propanoate is valued for its fruity aroma, reminiscent of pineapple and pear. The global flavor and fragrance market is projected to expand at a compound annual growth rate (CAGR) of over 4% in the coming years, positively impacting the demand for ethyl propanoate. The food and beverage sector, in particular, is a major consumer of this compound, using it in the production of artificial flavors and essences.
The pharmaceutical industry represents another significant market for ethyl propanoate. As an intermediate in drug synthesis, its demand is closely tied to the growth of the pharmaceutical sector. With the global pharmaceutical market expected to reach substantial values in the near future, the need for ethyl propanoate is likely to increase correspondingly.
In the agrochemical sector, ethyl propanoate serves as a precursor for various pesticides and herbicides. The growing emphasis on sustainable agriculture and increased food production to meet the needs of a rising global population is driving the demand for agrochemicals, subsequently boosting the market for ethyl propanoate.
The solvent industry also contributes to the market growth of ethyl propanoate. Its properties as a low-toxicity solvent make it attractive for use in paints, coatings, and cleaning products. The global solvents market is anticipated to grow steadily, further supporting the demand for ethyl propanoate.
Geographically, Asia-Pacific is emerging as a key market for ethyl propanoate, driven by rapid industrialization, growing population, and increasing disposable incomes. North America and Europe continue to be significant markets, particularly in the pharmaceutical and flavor industries.
The shift towards sustainable and green chemistry is influencing the ethyl propanoate market. Electrocatalytic reduction for chemical synthesis aligns well with this trend, offering a more environmentally friendly production method compared to traditional synthesis routes. This eco-friendly approach is likely to attract environmentally conscious consumers and industries, potentially expanding the market further.
However, the market faces challenges such as volatility in raw material prices and stringent regulations regarding chemical production and use. Despite these obstacles, the diverse applications and growing demand across multiple industries indicate a positive outlook for the ethyl propanoate market, particularly when produced through sustainable methods like electrocatalytic reduction.
In the flavor and fragrance industry, ethyl propanoate is valued for its fruity aroma, reminiscent of pineapple and pear. The global flavor and fragrance market is projected to expand at a compound annual growth rate (CAGR) of over 4% in the coming years, positively impacting the demand for ethyl propanoate. The food and beverage sector, in particular, is a major consumer of this compound, using it in the production of artificial flavors and essences.
The pharmaceutical industry represents another significant market for ethyl propanoate. As an intermediate in drug synthesis, its demand is closely tied to the growth of the pharmaceutical sector. With the global pharmaceutical market expected to reach substantial values in the near future, the need for ethyl propanoate is likely to increase correspondingly.
In the agrochemical sector, ethyl propanoate serves as a precursor for various pesticides and herbicides. The growing emphasis on sustainable agriculture and increased food production to meet the needs of a rising global population is driving the demand for agrochemicals, subsequently boosting the market for ethyl propanoate.
The solvent industry also contributes to the market growth of ethyl propanoate. Its properties as a low-toxicity solvent make it attractive for use in paints, coatings, and cleaning products. The global solvents market is anticipated to grow steadily, further supporting the demand for ethyl propanoate.
Geographically, Asia-Pacific is emerging as a key market for ethyl propanoate, driven by rapid industrialization, growing population, and increasing disposable incomes. North America and Europe continue to be significant markets, particularly in the pharmaceutical and flavor industries.
The shift towards sustainable and green chemistry is influencing the ethyl propanoate market. Electrocatalytic reduction for chemical synthesis aligns well with this trend, offering a more environmentally friendly production method compared to traditional synthesis routes. This eco-friendly approach is likely to attract environmentally conscious consumers and industries, potentially expanding the market further.
However, the market faces challenges such as volatility in raw material prices and stringent regulations regarding chemical production and use. Despite these obstacles, the diverse applications and growing demand across multiple industries indicate a positive outlook for the ethyl propanoate market, particularly when produced through sustainable methods like electrocatalytic reduction.
Current Challenges in Electrocatalytic Reduction
The electrocatalytic reduction of ethyl propanoate for chemical synthesis faces several significant challenges that hinder its widespread industrial application. One of the primary obstacles is the low selectivity of the reduction process. The reaction often produces a mixture of products, including unwanted side reactions, which reduces the overall efficiency and yield of the desired compound. This lack of selectivity is particularly problematic when aiming for high-purity products required in pharmaceutical or fine chemical industries.
Another major challenge is the high overpotential required for the reduction reaction. The substantial energy input needed to overcome this overpotential increases the process's overall energy consumption, making it less economically viable and environmentally sustainable. Developing catalysts that can lower this overpotential while maintaining high activity and selectivity remains a key research focus.
The stability of electrocatalysts under reaction conditions poses another significant hurdle. Many catalysts suffer from deactivation or degradation during extended operation, leading to decreased performance over time. This instability not only affects the reaction efficiency but also increases operational costs due to the need for frequent catalyst replacement or regeneration.
Mass transfer limitations in the electrochemical cell design present additional challenges. The efficient transport of reactants to the electrode surface and the removal of products are crucial for maintaining high reaction rates. Current cell designs often struggle to achieve optimal mass transfer, particularly at larger scales, which can lead to reduced reaction rates and lower overall productivity.
The scalability of electrocatalytic reduction processes for ethyl propanoate is another critical issue. While laboratory-scale experiments may show promising results, translating these into industrially viable processes presents numerous engineering challenges. These include maintaining uniform current distribution, managing heat generation, and ensuring consistent product quality across larger reactor volumes.
Furthermore, the choice of electrode materials and electrolytes significantly impacts the reaction's efficiency and selectivity. Finding cost-effective, stable, and high-performance materials that can withstand the harsh electrochemical environment while promoting the desired reaction pathway remains an ongoing challenge in the field.
Lastly, the integration of electrocatalytic reduction processes into existing chemical production workflows presents logistical and economic challenges. Adapting current infrastructure to accommodate electrochemical processes and ensuring their compatibility with downstream processing steps requires significant investment and process redesign, which can be a barrier to industrial adoption.
Another major challenge is the high overpotential required for the reduction reaction. The substantial energy input needed to overcome this overpotential increases the process's overall energy consumption, making it less economically viable and environmentally sustainable. Developing catalysts that can lower this overpotential while maintaining high activity and selectivity remains a key research focus.
The stability of electrocatalysts under reaction conditions poses another significant hurdle. Many catalysts suffer from deactivation or degradation during extended operation, leading to decreased performance over time. This instability not only affects the reaction efficiency but also increases operational costs due to the need for frequent catalyst replacement or regeneration.
Mass transfer limitations in the electrochemical cell design present additional challenges. The efficient transport of reactants to the electrode surface and the removal of products are crucial for maintaining high reaction rates. Current cell designs often struggle to achieve optimal mass transfer, particularly at larger scales, which can lead to reduced reaction rates and lower overall productivity.
The scalability of electrocatalytic reduction processes for ethyl propanoate is another critical issue. While laboratory-scale experiments may show promising results, translating these into industrially viable processes presents numerous engineering challenges. These include maintaining uniform current distribution, managing heat generation, and ensuring consistent product quality across larger reactor volumes.
Furthermore, the choice of electrode materials and electrolytes significantly impacts the reaction's efficiency and selectivity. Finding cost-effective, stable, and high-performance materials that can withstand the harsh electrochemical environment while promoting the desired reaction pathway remains an ongoing challenge in the field.
Lastly, the integration of electrocatalytic reduction processes into existing chemical production workflows presents logistical and economic challenges. Adapting current infrastructure to accommodate electrochemical processes and ensuring their compatibility with downstream processing steps requires significant investment and process redesign, which can be a barrier to industrial adoption.
Existing Electrocatalytic Reduction Methods
01 Electrocatalysts for ethyl propanoate reduction
Various electrocatalysts can be used for the reduction of ethyl propanoate. These catalysts may include metal-based materials, such as copper, nickel, or palladium, as well as carbon-based materials. The choice of catalyst significantly affects the efficiency and selectivity of the reduction process.- Electrocatalysts for ethyl propanoate reduction: Various electrocatalysts are used for the reduction of ethyl propanoate. These catalysts can include metal-based materials, alloys, or composite structures designed to facilitate the electrochemical reduction process. The choice of catalyst significantly affects the efficiency and selectivity of the reduction reaction.
- Reaction conditions optimization: Optimizing reaction conditions is crucial for efficient ethyl propanoate electrocatalytic reduction. This includes adjusting parameters such as temperature, pressure, pH, and electrolyte composition. Proper control of these conditions can enhance reaction rates, improve product yields, and minimize unwanted side reactions.
- Electrode materials and design: The selection and design of electrode materials play a vital role in the electrocatalytic reduction of ethyl propanoate. Researchers explore various electrode configurations, including nanostructured materials, porous electrodes, and composite electrodes, to improve the reaction efficiency and selectivity.
- Process integration and scale-up: Integrating the electrocatalytic reduction of ethyl propanoate into larger chemical processes and scaling up the reaction for industrial applications are important considerations. This involves addressing challenges related to heat management, mass transfer limitations, and maintaining catalyst stability during prolonged operation.
- Product separation and purification: Developing efficient methods for separating and purifying the products of ethyl propanoate electrocatalytic reduction is essential for practical applications. This may involve techniques such as distillation, extraction, or membrane separation to isolate the desired products from the reaction mixture and unreacted starting materials.
02 Reaction conditions optimization
Optimizing reaction conditions is crucial for efficient ethyl propanoate electrocatalytic reduction. This includes controlling parameters such as temperature, pressure, pH, and electrolyte composition. Proper adjustment of these conditions can enhance reaction rates and product yields.Expand Specific Solutions03 Electrode materials and design
The choice of electrode materials and their design play a significant role in the electrocatalytic reduction of ethyl propanoate. Novel electrode configurations, such as nanostructured or porous electrodes, can improve the reaction efficiency by increasing the active surface area and enhancing mass transfer.Expand Specific Solutions04 Product separation and purification
Developing efficient methods for separating and purifying the products of ethyl propanoate electrocatalytic reduction is essential. This may involve techniques such as distillation, extraction, or membrane separation to isolate the desired products from the reaction mixture.Expand Specific Solutions05 Process integration and scale-up
Integrating the electrocatalytic reduction of ethyl propanoate into larger chemical processes and scaling up the reaction for industrial applications are important considerations. This involves addressing challenges related to heat management, mass transfer limitations, and maintaining catalyst stability at larger scales.Expand Specific Solutions
Key Players in Electrocatalytic Synthesis Industry
The electrocatalytic reduction of ethyl propanoate for chemical synthesis is an emerging field with growing interest from both academia and industry. The market is in its early stages of development, with research efforts primarily focused on improving catalytic efficiency and selectivity. While the market size is currently limited, it shows potential for growth as sustainable chemical synthesis methods gain traction. The technology's maturity is still evolving, with key players like Xiamen University, Resonac Corp., and BASF Corp. leading research efforts. Universities such as King Fahd University of Petroleum & Minerals and Nankai University are also contributing significantly to advancing the fundamental understanding of this process. As the technology progresses, collaboration between academic institutions and industrial partners will be crucial for scaling up and commercializing this sustainable approach to chemical synthesis.
BASF Corp.
Technical Solution: BASF has developed an innovative electrocatalytic reduction process for the synthesis of ethyl propanoate. Their approach utilizes a copper-based catalyst system with high selectivity and efficiency[1]. The process operates at ambient temperature and pressure, significantly reducing energy consumption compared to traditional methods[2]. BASF's electrocatalytic cell design incorporates a novel membrane electrode assembly, allowing for continuous production and easy scale-up[3]. The company has also implemented advanced process control systems to optimize reaction conditions and maintain product quality[4].
Strengths: High selectivity, energy efficiency, and scalability. Weaknesses: Potential high initial investment costs and reliance on specific catalyst materials.
Nankai University
Technical Solution: Researchers at Nankai University have pioneered a novel approach to the electrocatalytic reduction of ethyl propanoate using graphene-supported metal nanoparticles[5]. Their method employs a unique combination of palladium and copper nanoparticles dispersed on reduced graphene oxide sheets, achieving high catalytic activity and selectivity[6]. The team has optimized the electrode structure to enhance mass transfer and electron conductivity, resulting in improved reaction kinetics[7]. Additionally, they have developed an in-situ spectroscopic technique to monitor the reaction progress and elucidate the mechanism[8].
Strengths: High catalytic activity, mechanistic insights, and potential for further optimization. Weaknesses: Possible challenges in large-scale production and catalyst stability over extended periods.
Core Innovations in Electrocatalytic Reduction
Method for Electrocatalytic Reduction using Au Nanoparticles Tuned or Optimized for Reduction of CO2 to CO
PatentInactiveUS20160230295A1
Innovation
- The use of gold nanoparticles with a diameter of approximately 8 nm, supported on Ketjen carbon, which presents a surface structure with a higher density of CO-converting edge sites and fewer hydrogen-evolving corner sites, facilitating efficient CO2 reduction to carbon monoxide in an alkaline solution.
Process for the carbonylation of an ethylenically unsaturated compound and catalyst therefore
PatentInactiveUS7202193B2
Innovation
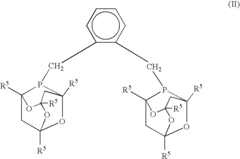
- A process involving a catalyst comprising a group VIII metal, a bidentate diphosphine ligand of specific formula, and a source of anions, where the diphosphine ligand includes a 2-phospha-tricyclo[3.3.1.1{3,7}]-decyl group or derivative, and the aromatic group is linked via alkylene groups, enhancing reaction efficiency.
Green Chemistry Aspects of Electrocatalytic Reduction
The electrocatalytic reduction of ethyl propanoate for chemical synthesis aligns well with the principles of green chemistry, offering a more sustainable approach to traditional synthetic methods. This process significantly reduces the environmental impact of chemical production by utilizing renewable electricity as the primary energy source, thereby minimizing reliance on fossil fuels and reducing carbon emissions.
One of the key green chemistry aspects of this approach is the use of mild reaction conditions. Electrocatalytic reduction typically occurs at room temperature and atmospheric pressure, eliminating the need for energy-intensive heating or pressurization. This not only reduces energy consumption but also enhances safety in chemical processes.
The selectivity of electrocatalytic reduction is another crucial green chemistry feature. By carefully controlling the electrode potential and catalyst design, it is possible to achieve high selectivity towards desired products, minimizing the formation of unwanted by-products. This increased selectivity leads to improved atom economy and reduces the need for extensive purification steps, further reducing waste and resource consumption.
Electrocatalytic reduction often employs water as a solvent or co-solvent, replacing harmful organic solvents commonly used in traditional synthetic methods. This shift towards aqueous reaction media significantly reduces the environmental and health risks associated with solvent use and disposal.
The catalysts used in electrocatalytic reduction are typically heterogeneous, allowing for easy separation and potential reuse. This characteristic aligns with the green chemistry principle of designing for reuse and recycling, reducing the overall material consumption in chemical processes.
Furthermore, the electrocatalytic approach enables the use of renewable feedstocks. In the case of ethyl propanoate reduction, the starting material can be derived from bio-based sources, promoting the use of sustainable raw materials in chemical synthesis.
The scalability of electrocatalytic processes is another green chemistry advantage. These systems can be easily scaled up or down, allowing for on-demand production and reducing the need for large-scale storage of potentially hazardous chemicals.
Lastly, the integration of electrocatalytic reduction into flow chemistry setups further enhances its green chemistry profile. Continuous flow processes offer improved heat and mass transfer, leading to higher efficiency, reduced reaction times, and minimized waste generation.
One of the key green chemistry aspects of this approach is the use of mild reaction conditions. Electrocatalytic reduction typically occurs at room temperature and atmospheric pressure, eliminating the need for energy-intensive heating or pressurization. This not only reduces energy consumption but also enhances safety in chemical processes.
The selectivity of electrocatalytic reduction is another crucial green chemistry feature. By carefully controlling the electrode potential and catalyst design, it is possible to achieve high selectivity towards desired products, minimizing the formation of unwanted by-products. This increased selectivity leads to improved atom economy and reduces the need for extensive purification steps, further reducing waste and resource consumption.
Electrocatalytic reduction often employs water as a solvent or co-solvent, replacing harmful organic solvents commonly used in traditional synthetic methods. This shift towards aqueous reaction media significantly reduces the environmental and health risks associated with solvent use and disposal.
The catalysts used in electrocatalytic reduction are typically heterogeneous, allowing for easy separation and potential reuse. This characteristic aligns with the green chemistry principle of designing for reuse and recycling, reducing the overall material consumption in chemical processes.
Furthermore, the electrocatalytic approach enables the use of renewable feedstocks. In the case of ethyl propanoate reduction, the starting material can be derived from bio-based sources, promoting the use of sustainable raw materials in chemical synthesis.
The scalability of electrocatalytic processes is another green chemistry advantage. These systems can be easily scaled up or down, allowing for on-demand production and reducing the need for large-scale storage of potentially hazardous chemicals.
Lastly, the integration of electrocatalytic reduction into flow chemistry setups further enhances its green chemistry profile. Continuous flow processes offer improved heat and mass transfer, leading to higher efficiency, reduced reaction times, and minimized waste generation.
Economic Feasibility of Electrocatalytic Synthesis
The economic feasibility of electrocatalytic synthesis for ethyl propanoate production is a critical factor in determining its potential for industrial adoption. This approach offers several advantages over traditional chemical synthesis methods, potentially leading to significant cost savings and improved sustainability.
One of the primary economic benefits of electrocatalytic reduction is the potential for reduced energy consumption. Traditional synthesis methods often require high temperatures and pressures, resulting in substantial energy costs. In contrast, electrocatalytic processes can operate under milder conditions, potentially lowering overall energy requirements and associated expenses.
Raw material costs may also be reduced through electrocatalytic synthesis. This method can utilize more readily available and less expensive starting materials, such as carbon dioxide and water, instead of petroleum-based feedstocks. This shift could lead to decreased dependency on volatile petrochemical markets and more stable input costs.
The electrocatalytic approach also offers the possibility of improved product selectivity and yield. By fine-tuning the catalysts and reaction conditions, it may be possible to achieve higher conversion rates and reduce unwanted by-products. This increased efficiency could translate to lower production costs and reduced waste management expenses.
Capital investment requirements for electrocatalytic synthesis facilities may initially be higher than traditional chemical plants due to the specialized equipment needed. However, the potential for process intensification and smaller plant footprints could offset these costs in the long term. Additionally, the modular nature of electrocatalytic systems may allow for more flexible scaling of production capacity.
Operating costs for electrocatalytic synthesis could be lower due to reduced need for high-pressure equipment, complex separation processes, and extensive safety measures often associated with traditional chemical synthesis. This simplification of the production process may lead to lower maintenance costs and reduced downtime.
The economic viability of electrocatalytic synthesis is also influenced by external factors such as electricity prices and environmental regulations. As renewable energy sources become more prevalent and cost-effective, the economics of electrocatalytic processes are likely to improve further. Additionally, stricter environmental regulations may favor electrocatalytic methods due to their potential for reduced emissions and improved sustainability profile.
In conclusion, while the economic feasibility of electrocatalytic synthesis for ethyl propanoate production shows promise, a detailed cost-benefit analysis considering all aspects of production, from raw materials to final product distribution, is necessary to fully assess its viability compared to established methods.
One of the primary economic benefits of electrocatalytic reduction is the potential for reduced energy consumption. Traditional synthesis methods often require high temperatures and pressures, resulting in substantial energy costs. In contrast, electrocatalytic processes can operate under milder conditions, potentially lowering overall energy requirements and associated expenses.
Raw material costs may also be reduced through electrocatalytic synthesis. This method can utilize more readily available and less expensive starting materials, such as carbon dioxide and water, instead of petroleum-based feedstocks. This shift could lead to decreased dependency on volatile petrochemical markets and more stable input costs.
The electrocatalytic approach also offers the possibility of improved product selectivity and yield. By fine-tuning the catalysts and reaction conditions, it may be possible to achieve higher conversion rates and reduce unwanted by-products. This increased efficiency could translate to lower production costs and reduced waste management expenses.
Capital investment requirements for electrocatalytic synthesis facilities may initially be higher than traditional chemical plants due to the specialized equipment needed. However, the potential for process intensification and smaller plant footprints could offset these costs in the long term. Additionally, the modular nature of electrocatalytic systems may allow for more flexible scaling of production capacity.
Operating costs for electrocatalytic synthesis could be lower due to reduced need for high-pressure equipment, complex separation processes, and extensive safety measures often associated with traditional chemical synthesis. This simplification of the production process may lead to lower maintenance costs and reduced downtime.
The economic viability of electrocatalytic synthesis is also influenced by external factors such as electricity prices and environmental regulations. As renewable energy sources become more prevalent and cost-effective, the economics of electrocatalytic processes are likely to improve further. Additionally, stricter environmental regulations may favor electrocatalytic methods due to their potential for reduced emissions and improved sustainability profile.
In conclusion, while the economic feasibility of electrocatalytic synthesis for ethyl propanoate production shows promise, a detailed cost-benefit analysis considering all aspects of production, from raw materials to final product distribution, is necessary to fully assess its viability compared to established methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!