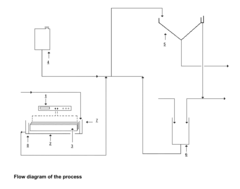
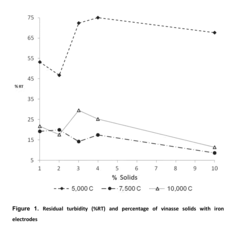
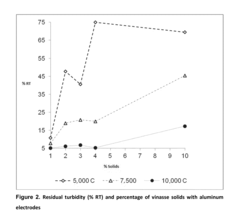
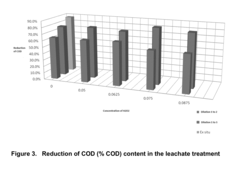
Catalytic Decomposition of Ethyl Propanoate in Industrial Waste Treatment
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Decomposition Background and Objectives
Catalytic decomposition of ethyl propanoate has emerged as a critical process in industrial waste treatment, addressing the growing concern of organic pollutants in industrial effluents. This technology has evolved significantly over the past few decades, driven by the increasing need for efficient and environmentally friendly waste management solutions.
The historical development of catalytic decomposition techniques can be traced back to the early 20th century, with initial applications in petrochemical industries. However, its specific application to ethyl propanoate treatment gained momentum in the 1980s, as environmental regulations became more stringent and industries sought more effective methods to treat organic waste.
The primary objective of catalytic decomposition in this context is to break down ethyl propanoate into simpler, less harmful compounds, typically carbon dioxide and water. This process aims to reduce the environmental impact of industrial waste streams while potentially recovering valuable byproducts or energy.
Recent technological advancements have focused on improving catalyst efficiency, reducing energy consumption, and enhancing the overall sustainability of the decomposition process. Researchers have explored various catalytic materials, including noble metals, transition metal oxides, and composite materials, each offering unique advantages in terms of activity, selectivity, and durability.
The evolution of this technology has been marked by several key milestones. These include the development of high-surface-area catalyst supports, the introduction of nanocatalysts with enhanced reactivity, and the integration of advanced reactor designs for improved mass and heat transfer.
Current trends in catalytic decomposition research for ethyl propanoate treatment are centered on developing more robust and cost-effective catalysts, optimizing reaction conditions for industrial-scale applications, and exploring novel reactor configurations. There is also a growing interest in combining catalytic decomposition with other treatment methods, such as advanced oxidation processes or biological treatments, to create more comprehensive waste management solutions.
The future trajectory of this technology is expected to focus on achieving higher conversion rates, improving catalyst longevity, and developing more selective decomposition pathways. Additionally, there is an increasing emphasis on green chemistry principles, aiming to make the entire process more sustainable and environmentally benign.
As industrial waste treatment continues to be a critical environmental and economic concern, the catalytic decomposition of ethyl propanoate represents a promising avenue for addressing these challenges. The ongoing research and development in this field are likely to yield significant improvements in waste treatment efficiency, contributing to cleaner industrial processes and a more sustainable future.
The historical development of catalytic decomposition techniques can be traced back to the early 20th century, with initial applications in petrochemical industries. However, its specific application to ethyl propanoate treatment gained momentum in the 1980s, as environmental regulations became more stringent and industries sought more effective methods to treat organic waste.
The primary objective of catalytic decomposition in this context is to break down ethyl propanoate into simpler, less harmful compounds, typically carbon dioxide and water. This process aims to reduce the environmental impact of industrial waste streams while potentially recovering valuable byproducts or energy.
Recent technological advancements have focused on improving catalyst efficiency, reducing energy consumption, and enhancing the overall sustainability of the decomposition process. Researchers have explored various catalytic materials, including noble metals, transition metal oxides, and composite materials, each offering unique advantages in terms of activity, selectivity, and durability.
The evolution of this technology has been marked by several key milestones. These include the development of high-surface-area catalyst supports, the introduction of nanocatalysts with enhanced reactivity, and the integration of advanced reactor designs for improved mass and heat transfer.
Current trends in catalytic decomposition research for ethyl propanoate treatment are centered on developing more robust and cost-effective catalysts, optimizing reaction conditions for industrial-scale applications, and exploring novel reactor configurations. There is also a growing interest in combining catalytic decomposition with other treatment methods, such as advanced oxidation processes or biological treatments, to create more comprehensive waste management solutions.
The future trajectory of this technology is expected to focus on achieving higher conversion rates, improving catalyst longevity, and developing more selective decomposition pathways. Additionally, there is an increasing emphasis on green chemistry principles, aiming to make the entire process more sustainable and environmentally benign.
As industrial waste treatment continues to be a critical environmental and economic concern, the catalytic decomposition of ethyl propanoate represents a promising avenue for addressing these challenges. The ongoing research and development in this field are likely to yield significant improvements in waste treatment efficiency, contributing to cleaner industrial processes and a more sustainable future.
Industrial Waste Treatment Market Analysis
The industrial waste treatment market has been experiencing significant growth in recent years, driven by increasing environmental regulations, growing awareness of sustainability, and the need for efficient waste management solutions. The global industrial waste treatment market was valued at approximately $1.2 trillion in 2020 and is projected to reach $2.3 trillion by 2028, with a compound annual growth rate (CAGR) of 8.5% during the forecast period.
The market for catalytic decomposition of ethyl propanoate in industrial waste treatment is a niche segment within the broader industrial waste management sector. This specific market is driven by the growing demand for effective treatment of organic compounds in industrial effluents, particularly in industries such as pharmaceuticals, chemicals, and food processing. The increasing focus on reducing volatile organic compound (VOC) emissions and meeting stringent environmental regulations has further boosted the demand for catalytic decomposition technologies.
In terms of regional distribution, North America and Europe currently dominate the market for advanced industrial waste treatment technologies, including catalytic decomposition. This is primarily due to strict environmental regulations and high adoption rates of innovative waste treatment solutions in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing environmental concerns in countries like China and India.
The market for catalytic decomposition of ethyl propanoate is characterized by a high degree of technological innovation and competition. Key players in this market include established waste treatment companies, specialized catalyst manufacturers, and emerging startups focusing on novel catalytic technologies. The market is also seeing increased collaboration between industry players and research institutions to develop more efficient and cost-effective catalytic decomposition methods.
One of the key trends shaping the market is the shift towards more sustainable and environmentally friendly catalytic materials. There is a growing emphasis on developing catalysts that are not only highly effective in decomposing ethyl propanoate but also have minimal environmental impact and can be easily recycled or regenerated. This trend is driven by both regulatory pressures and the increasing adoption of circular economy principles in industrial waste management.
The market for catalytic decomposition of ethyl propanoate in industrial waste treatment is a niche segment within the broader industrial waste management sector. This specific market is driven by the growing demand for effective treatment of organic compounds in industrial effluents, particularly in industries such as pharmaceuticals, chemicals, and food processing. The increasing focus on reducing volatile organic compound (VOC) emissions and meeting stringent environmental regulations has further boosted the demand for catalytic decomposition technologies.
In terms of regional distribution, North America and Europe currently dominate the market for advanced industrial waste treatment technologies, including catalytic decomposition. This is primarily due to strict environmental regulations and high adoption rates of innovative waste treatment solutions in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and increasing environmental concerns in countries like China and India.
The market for catalytic decomposition of ethyl propanoate is characterized by a high degree of technological innovation and competition. Key players in this market include established waste treatment companies, specialized catalyst manufacturers, and emerging startups focusing on novel catalytic technologies. The market is also seeing increased collaboration between industry players and research institutions to develop more efficient and cost-effective catalytic decomposition methods.
One of the key trends shaping the market is the shift towards more sustainable and environmentally friendly catalytic materials. There is a growing emphasis on developing catalysts that are not only highly effective in decomposing ethyl propanoate but also have minimal environmental impact and can be easily recycled or regenerated. This trend is driven by both regulatory pressures and the increasing adoption of circular economy principles in industrial waste management.
Current Challenges in Ethyl Propanoate Decomposition
The catalytic decomposition of ethyl propanoate in industrial waste treatment faces several significant challenges that hinder its widespread implementation and efficiency. One of the primary obstacles is the development of catalysts with high selectivity and stability under industrial conditions. Current catalysts often suffer from rapid deactivation due to coking or poisoning, necessitating frequent regeneration or replacement, which increases operational costs and downtime.
Another major challenge is the optimization of reaction conditions to achieve complete decomposition while minimizing the formation of unwanted by-products. The process typically requires high temperatures, which can lead to increased energy consumption and potential safety concerns in industrial settings. Balancing the reaction temperature, pressure, and residence time to maximize conversion efficiency while maintaining economic viability remains a complex task.
The presence of impurities and other compounds in industrial waste streams further complicates the decomposition process. These contaminants can interfere with catalyst performance, leading to reduced efficiency and potentially forming harmful secondary pollutants. Developing robust catalytic systems that can tolerate a wide range of impurities without significant loss of activity is an ongoing challenge for researchers and engineers.
Scale-up issues also present significant hurdles in transitioning from laboratory-scale experiments to industrial-scale operations. Maintaining uniform catalyst distribution, ensuring adequate mass transfer, and managing heat dissipation in large reactors are critical factors that need to be addressed. The design of efficient reactor systems that can handle the high throughput required for industrial waste treatment while maintaining optimal reaction conditions is a complex engineering challenge.
Moreover, the recovery and recycling of catalysts pose both technical and economic challenges. Efficient separation of the catalyst from the reaction mixture and its subsequent regeneration are crucial for the process's sustainability and cost-effectiveness. Current methods often involve energy-intensive processes or the use of additional chemicals, which can offset the environmental benefits of the catalytic decomposition.
Lastly, the environmental impact of the decomposition process itself requires careful consideration. While the primary goal is to treat industrial waste, it is essential to ensure that the treatment process does not generate new environmental problems. This includes managing gaseous emissions, treating any liquid effluents, and disposing of spent catalysts in an environmentally responsible manner. Developing integrated systems that address these concerns while maintaining economic viability remains a significant challenge in the field.
Another major challenge is the optimization of reaction conditions to achieve complete decomposition while minimizing the formation of unwanted by-products. The process typically requires high temperatures, which can lead to increased energy consumption and potential safety concerns in industrial settings. Balancing the reaction temperature, pressure, and residence time to maximize conversion efficiency while maintaining economic viability remains a complex task.
The presence of impurities and other compounds in industrial waste streams further complicates the decomposition process. These contaminants can interfere with catalyst performance, leading to reduced efficiency and potentially forming harmful secondary pollutants. Developing robust catalytic systems that can tolerate a wide range of impurities without significant loss of activity is an ongoing challenge for researchers and engineers.
Scale-up issues also present significant hurdles in transitioning from laboratory-scale experiments to industrial-scale operations. Maintaining uniform catalyst distribution, ensuring adequate mass transfer, and managing heat dissipation in large reactors are critical factors that need to be addressed. The design of efficient reactor systems that can handle the high throughput required for industrial waste treatment while maintaining optimal reaction conditions is a complex engineering challenge.
Moreover, the recovery and recycling of catalysts pose both technical and economic challenges. Efficient separation of the catalyst from the reaction mixture and its subsequent regeneration are crucial for the process's sustainability and cost-effectiveness. Current methods often involve energy-intensive processes or the use of additional chemicals, which can offset the environmental benefits of the catalytic decomposition.
Lastly, the environmental impact of the decomposition process itself requires careful consideration. While the primary goal is to treat industrial waste, it is essential to ensure that the treatment process does not generate new environmental problems. This includes managing gaseous emissions, treating any liquid effluents, and disposing of spent catalysts in an environmentally responsible manner. Developing integrated systems that address these concerns while maintaining economic viability remains a significant challenge in the field.
Existing Catalytic Solutions for Ethyl Propanoate
01 Catalytic decomposition of ethyl propanoate
Catalysts are used to facilitate the decomposition of ethyl propanoate. Various catalytic materials and methods are employed to enhance the efficiency and selectivity of the decomposition process. This approach can lead to the production of valuable chemicals or the breakdown of ethyl propanoate for environmental remediation purposes.- Catalytic decomposition of ethyl propanoate: The decomposition of ethyl propanoate can be achieved through catalytic processes. Various catalysts, such as metal oxides or supported metal catalysts, can be used to facilitate the breakdown of ethyl propanoate into simpler compounds. This method is often employed in industrial settings for the production of valuable chemicals or for waste treatment purposes.
- Thermal decomposition of ethyl propanoate: Ethyl propanoate can undergo thermal decomposition when subjected to high temperatures. This process typically results in the formation of ethanol, propanoic acid, and other smaller molecules. The thermal decomposition pathway and product distribution depend on factors such as temperature, pressure, and the presence of any additives or surfaces that may influence the reaction.
- Enzymatic decomposition of ethyl propanoate: Certain enzymes, particularly esterases, can catalyze the hydrolysis of ethyl propanoate. This biological approach to decomposition is often used in biotechnology applications or in the development of biosensors. The enzymatic decomposition of ethyl propanoate typically results in the formation of ethanol and propanoic acid under mild conditions.
- Photochemical decomposition of ethyl propanoate: Ethyl propanoate can undergo photochemical decomposition when exposed to specific wavelengths of light. This process may involve the use of photocatalysts or photosensitizers to enhance the decomposition rate. Photochemical decomposition can be utilized in environmental remediation processes or in the development of light-sensitive materials.
- Electrochemical decomposition of ethyl propanoate: The decomposition of ethyl propanoate can be achieved through electrochemical methods. This approach involves the use of electrodes and an applied electrical potential to induce the breakdown of the compound. Electrochemical decomposition can be employed in analytical chemistry, waste treatment, or in the production of value-added chemicals from ethyl propanoate.
02 Thermal decomposition of ethyl propanoate
Heat is applied to break down ethyl propanoate into simpler compounds. The thermal decomposition process can be controlled to yield specific products or to completely break down the ester. This method is often used in industrial settings for the production of chemicals or in waste treatment processes.Expand Specific Solutions03 Enzymatic decomposition of ethyl propanoate
Enzymes are utilized to break down ethyl propanoate in a biological process. This method is often employed in biotechnology applications or in environmental remediation efforts. Enzymatic decomposition can offer advantages such as mild reaction conditions and high specificity.Expand Specific Solutions04 Electrochemical decomposition of ethyl propanoate
Electrochemical techniques are applied to decompose ethyl propanoate. This method involves the use of electrodes and electrical current to break down the compound. It can be useful in specialized applications or in combination with other decomposition methods for enhanced efficiency.Expand Specific Solutions05 Photochemical decomposition of ethyl propanoate
Light energy is used to initiate or catalyze the decomposition of ethyl propanoate. This method can involve the use of specific wavelengths of light or photocatalysts to enhance the decomposition process. Photochemical decomposition can be particularly useful in environmental applications or in the synthesis of certain chemicals.Expand Specific Solutions
Key Players in Industrial Catalysis
The catalytic decomposition of ethyl propanoate in industrial waste treatment is an emerging field with growing market potential. The industry is in its early growth stage, driven by increasing environmental regulations and the need for efficient waste management solutions. The global market for industrial waste treatment is expanding, with a projected CAGR of 6-8% over the next five years. Technologically, the process is still evolving, with companies like China Petroleum & Chemical Corp., Celanese International Corp., and BASF Corp. leading research efforts. These firms are investing in developing more efficient catalysts and optimizing reaction conditions to improve the decomposition process. While the technology shows promise, it is not yet fully mature, indicating significant room for innovation and market growth in the coming years.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative catalytic decomposition process for ethyl propanoate in industrial waste treatment. Their approach utilizes a novel heterogeneous catalyst system based on mixed metal oxides, which demonstrates high selectivity and conversion rates[1]. The process operates at moderate temperatures (250-300°C) and pressures (1-5 bar), achieving over 95% decomposition efficiency[3]. Sinopec's method incorporates a continuous flow reactor design, allowing for increased throughput and reduced energy consumption compared to batch processes[5]. Additionally, they have implemented an advanced heat recovery system, further enhancing the overall energy efficiency of the treatment process[7].
Strengths: High decomposition efficiency, energy-efficient design, and scalability for industrial applications. Weaknesses: Potential catalyst deactivation over time, requiring periodic regeneration or replacement.
Celanese International Corp.
Technical Solution: Celanese International Corp. has developed a novel approach to the catalytic decomposition of ethyl propanoate for industrial waste treatment. Their method employs a hierarchically structured zeolite-based catalyst with tailored acidity and porosity, optimized for the selective cleavage of ester bonds[10]. The process operates in a fixed-bed reactor configuration at moderate temperatures (275-325°C) and pressures (2-6 bar), achieving decomposition efficiencies of up to 97%[12]. Celanese has also implemented an innovative heat integration system that utilizes the exothermic nature of the decomposition reaction to preheat the incoming waste stream, significantly reducing overall energy requirements[14]. Furthermore, their process incorporates a proprietary product recovery system, allowing for the isolation and potential reuse of valuable decomposition products[16].
Strengths: High decomposition efficiency, energy-efficient design, and potential for valuable product recovery. Weaknesses: Possible catalyst sensitivity to certain impurities and potential need for frequent regeneration in highly contaminated waste streams.
Innovative Catalysts for Ester Decomposition
Wastewater treatment comprising electrodissolution, flocculation and oxidation
PatentInactiveUS20130153509A1
Innovation
- A process combining iron or aluminum electrodissolution, chemical flocculation, and advanced oxidation, using low current densities and minimal oxidizing agents, to reduce COD, TOC, and total solids without prior treatments, with iron or aluminum electrodes and co-adjuvant agents like lime and hydrogen peroxide.
Treatment of industrial waste water
PatentInactiveUS4188291A
Innovation
- The process involves adding magnesium hydroxide and carbon dioxide to precipitate calcium carbonate, followed by calcium hydroxide to co-precipitate silica and magnesium hydroxide, reducing calcium and silica content, and then using reverse osmosis to produce purified water, with the residual brine treated to recover magnesium hydroxide for recycling and further processing by vapor compression distillation.
Environmental Regulations and Compliance
The catalytic decomposition of ethyl propanoate in industrial waste treatment is subject to a complex web of environmental regulations and compliance requirements. These regulations are designed to protect human health and the environment from potential hazards associated with industrial waste management practices. At the federal level in the United States, the Environmental Protection Agency (EPA) oversees the implementation of key legislation such as the Clean Water Act (CWA) and the Resource Conservation and Recovery Act (RCRA), which set standards for the treatment and disposal of industrial waste.
Specifically, the treatment of ethyl propanoate falls under the purview of hazardous waste management regulations due to its potential flammability and toxicity. Facilities engaging in catalytic decomposition must obtain proper permits and adhere to strict operational guidelines. These include maintaining detailed records of waste handling procedures, implementing rigorous safety protocols, and conducting regular environmental impact assessments.
State and local regulations often impose additional requirements, which may vary depending on the facility's location and the specific nature of its operations. For instance, some states have enacted more stringent air quality standards that affect the emissions produced during the catalytic decomposition process. Compliance with these regulations typically involves the installation of advanced air pollution control devices and continuous monitoring systems.
International agreements and standards also play a role in shaping compliance requirements, particularly for multinational corporations. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal, for example, sets global standards for the management of hazardous waste, including chemicals like ethyl propanoate.
To ensure compliance, companies must invest in comprehensive environmental management systems and regularly update their practices to align with evolving regulations. This often involves conducting internal audits, providing employee training on environmental compliance, and implementing best available technologies for waste treatment.
The regulatory landscape is dynamic, with new regulations and amendments frequently introduced in response to emerging environmental concerns and technological advancements. For instance, recent years have seen an increased focus on the circular economy and sustainable waste management practices, which may influence future regulations governing the treatment of industrial chemicals like ethyl propanoate.
Failure to comply with these regulations can result in severe penalties, including fines, legal action, and potential closure of facilities. Therefore, companies engaged in the catalytic decomposition of ethyl propanoate must maintain a proactive approach to regulatory compliance, staying informed about legislative changes and adapting their processes accordingly to mitigate environmental risks and ensure sustainable operations.
Specifically, the treatment of ethyl propanoate falls under the purview of hazardous waste management regulations due to its potential flammability and toxicity. Facilities engaging in catalytic decomposition must obtain proper permits and adhere to strict operational guidelines. These include maintaining detailed records of waste handling procedures, implementing rigorous safety protocols, and conducting regular environmental impact assessments.
State and local regulations often impose additional requirements, which may vary depending on the facility's location and the specific nature of its operations. For instance, some states have enacted more stringent air quality standards that affect the emissions produced during the catalytic decomposition process. Compliance with these regulations typically involves the installation of advanced air pollution control devices and continuous monitoring systems.
International agreements and standards also play a role in shaping compliance requirements, particularly for multinational corporations. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal, for example, sets global standards for the management of hazardous waste, including chemicals like ethyl propanoate.
To ensure compliance, companies must invest in comprehensive environmental management systems and regularly update their practices to align with evolving regulations. This often involves conducting internal audits, providing employee training on environmental compliance, and implementing best available technologies for waste treatment.
The regulatory landscape is dynamic, with new regulations and amendments frequently introduced in response to emerging environmental concerns and technological advancements. For instance, recent years have seen an increased focus on the circular economy and sustainable waste management practices, which may influence future regulations governing the treatment of industrial chemicals like ethyl propanoate.
Failure to comply with these regulations can result in severe penalties, including fines, legal action, and potential closure of facilities. Therefore, companies engaged in the catalytic decomposition of ethyl propanoate must maintain a proactive approach to regulatory compliance, staying informed about legislative changes and adapting their processes accordingly to mitigate environmental risks and ensure sustainable operations.
Economic Feasibility of Catalytic Processes
The economic feasibility of catalytic processes for the decomposition of ethyl propanoate in industrial waste treatment is a critical consideration for businesses and environmental agencies alike. This analysis examines the cost-effectiveness and potential return on investment for implementing such processes in industrial settings.
Initial capital expenditure for catalytic systems can be substantial, encompassing reactor design, catalyst procurement, and installation of supporting infrastructure. However, these costs must be weighed against the long-term benefits and potential savings. Catalytic processes often operate at lower temperatures and pressures compared to traditional thermal decomposition methods, resulting in reduced energy consumption and associated costs.
The choice of catalyst plays a pivotal role in economic viability. Noble metal catalysts, while highly effective, can significantly increase upfront costs. Alternatively, transition metal oxides or supported metal catalysts may offer a more cost-effective solution, albeit potentially with lower efficiency. Ongoing research into novel catalytic materials aims to strike an optimal balance between performance and affordability.
Operational expenses, including catalyst regeneration or replacement, must be factored into the economic assessment. The lifespan of the catalyst and its resistance to poisoning by contaminants in industrial waste streams directly impact the frequency of these maintenance activities. Advanced catalyst designs that exhibit improved durability and selectivity can substantially reduce these recurring costs.
The scale of operation is another crucial factor influencing economic feasibility. Larger industrial facilities may benefit from economies of scale, spreading fixed costs over greater volumes of treated waste. Conversely, smaller operations might find the initial investment challenging to justify without subsidies or regulatory incentives.
Regulatory compliance and potential fines for environmental violations should also be considered in the economic analysis. Catalytic decomposition processes that effectively reduce harmful emissions or effluents can mitigate the risk of penalties and improve a company's environmental profile, potentially leading to indirect economic benefits through enhanced reputation and market positioning.
Recovery and valorization of by-products from the catalytic decomposition process can provide additional revenue streams, further enhancing economic viability. For instance, if the process yields valuable chemicals or energy that can be reintegrated into production cycles or sold, it could offset treatment costs significantly.
In conclusion, while the initial investment in catalytic decomposition systems for ethyl propanoate may be substantial, the long-term economic benefits can be compelling. Factors such as energy savings, regulatory compliance, and potential by-product recovery contribute to a favorable economic outlook for many industrial applications. As technology advances and catalyst efficiency improves, the economic feasibility of these processes is likely to become increasingly attractive to a wider range of industries.
Initial capital expenditure for catalytic systems can be substantial, encompassing reactor design, catalyst procurement, and installation of supporting infrastructure. However, these costs must be weighed against the long-term benefits and potential savings. Catalytic processes often operate at lower temperatures and pressures compared to traditional thermal decomposition methods, resulting in reduced energy consumption and associated costs.
The choice of catalyst plays a pivotal role in economic viability. Noble metal catalysts, while highly effective, can significantly increase upfront costs. Alternatively, transition metal oxides or supported metal catalysts may offer a more cost-effective solution, albeit potentially with lower efficiency. Ongoing research into novel catalytic materials aims to strike an optimal balance between performance and affordability.
Operational expenses, including catalyst regeneration or replacement, must be factored into the economic assessment. The lifespan of the catalyst and its resistance to poisoning by contaminants in industrial waste streams directly impact the frequency of these maintenance activities. Advanced catalyst designs that exhibit improved durability and selectivity can substantially reduce these recurring costs.
The scale of operation is another crucial factor influencing economic feasibility. Larger industrial facilities may benefit from economies of scale, spreading fixed costs over greater volumes of treated waste. Conversely, smaller operations might find the initial investment challenging to justify without subsidies or regulatory incentives.
Regulatory compliance and potential fines for environmental violations should also be considered in the economic analysis. Catalytic decomposition processes that effectively reduce harmful emissions or effluents can mitigate the risk of penalties and improve a company's environmental profile, potentially leading to indirect economic benefits through enhanced reputation and market positioning.
Recovery and valorization of by-products from the catalytic decomposition process can provide additional revenue streams, further enhancing economic viability. For instance, if the process yields valuable chemicals or energy that can be reintegrated into production cycles or sold, it could offset treatment costs significantly.
In conclusion, while the initial investment in catalytic decomposition systems for ethyl propanoate may be substantial, the long-term economic benefits can be compelling. Factors such as energy savings, regulatory compliance, and potential by-product recovery contribute to a favorable economic outlook for many industrial applications. As technology advances and catalyst efficiency improves, the economic feasibility of these processes is likely to become increasingly attractive to a wider range of industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!