Ethyl Propanoate for De-Aromatization in Chemical Processes
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Background and Objectives
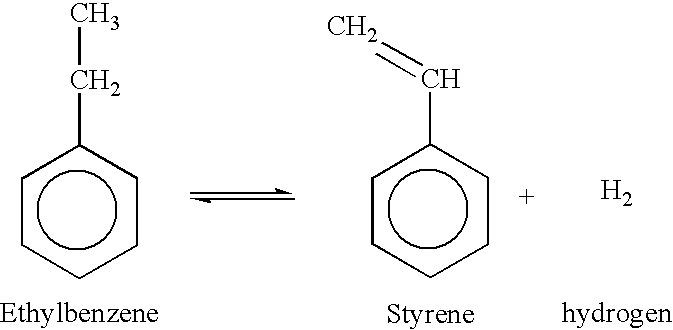
Ethyl propanoate, also known as ethyl propionate, is an organic compound with the molecular formula C5H10O2. It is a colorless liquid with a fruity odor, commonly used as a flavoring agent in the food industry. In recent years, its potential application in chemical processes, particularly for de-aromatization, has gained significant attention from researchers and industry professionals.
The development of ethyl propanoate as a de-aromatization agent stems from the growing need for more efficient and environmentally friendly processes in the chemical industry. Traditional de-aromatization methods often involve harsh conditions, toxic solvents, or energy-intensive procedures, which can lead to environmental concerns and increased production costs. As a result, there has been a push towards finding alternative solutions that can achieve similar or better results while minimizing negative impacts.
Ethyl propanoate's unique chemical properties make it a promising candidate for de-aromatization applications. Its relatively low boiling point, high solvency power, and low toxicity offer advantages over conventional solvents used in aromatic compound removal. Additionally, its biodegradability and potential for recovery and reuse align well with the principles of green chemistry and sustainable industrial practices.
The evolution of ethyl propanoate in chemical processes can be traced back to its initial use as a solvent and extracting agent in various industries. However, its specific application in de-aromatization is a more recent development, driven by the need for innovative solutions in petrochemical refining, pharmaceutical manufacturing, and environmental remediation.
The primary objective of researching ethyl propanoate for de-aromatization is to develop a comprehensive understanding of its mechanisms, efficacy, and potential applications across different chemical processes. This includes investigating its selectivity towards aromatic compounds, optimizing reaction conditions, and exploring synergistic effects with other solvents or catalysts.
Furthermore, the research aims to address several key challenges associated with de-aromatization processes. These include improving the efficiency of aromatic compound removal, reducing energy consumption, minimizing waste generation, and enhancing the overall sustainability of chemical processes. By focusing on ethyl propanoate, researchers hope to overcome these challenges and establish new benchmarks for de-aromatization technologies.
Another crucial aspect of this research is to evaluate the scalability and economic viability of ethyl propanoate-based de-aromatization processes. This involves assessing factors such as production costs, process integration, and potential impacts on existing industrial infrastructure. The ultimate goal is to develop practical, cost-effective solutions that can be readily adopted by various sectors of the chemical industry.
The development of ethyl propanoate as a de-aromatization agent stems from the growing need for more efficient and environmentally friendly processes in the chemical industry. Traditional de-aromatization methods often involve harsh conditions, toxic solvents, or energy-intensive procedures, which can lead to environmental concerns and increased production costs. As a result, there has been a push towards finding alternative solutions that can achieve similar or better results while minimizing negative impacts.
Ethyl propanoate's unique chemical properties make it a promising candidate for de-aromatization applications. Its relatively low boiling point, high solvency power, and low toxicity offer advantages over conventional solvents used in aromatic compound removal. Additionally, its biodegradability and potential for recovery and reuse align well with the principles of green chemistry and sustainable industrial practices.
The evolution of ethyl propanoate in chemical processes can be traced back to its initial use as a solvent and extracting agent in various industries. However, its specific application in de-aromatization is a more recent development, driven by the need for innovative solutions in petrochemical refining, pharmaceutical manufacturing, and environmental remediation.
The primary objective of researching ethyl propanoate for de-aromatization is to develop a comprehensive understanding of its mechanisms, efficacy, and potential applications across different chemical processes. This includes investigating its selectivity towards aromatic compounds, optimizing reaction conditions, and exploring synergistic effects with other solvents or catalysts.
Furthermore, the research aims to address several key challenges associated with de-aromatization processes. These include improving the efficiency of aromatic compound removal, reducing energy consumption, minimizing waste generation, and enhancing the overall sustainability of chemical processes. By focusing on ethyl propanoate, researchers hope to overcome these challenges and establish new benchmarks for de-aromatization technologies.
Another crucial aspect of this research is to evaluate the scalability and economic viability of ethyl propanoate-based de-aromatization processes. This involves assessing factors such as production costs, process integration, and potential impacts on existing industrial infrastructure. The ultimate goal is to develop practical, cost-effective solutions that can be readily adopted by various sectors of the chemical industry.
Market Analysis for De-Aromatization Agents
The market for de-aromatization agents, particularly ethyl propanoate, has shown significant growth in recent years due to increasing environmental regulations and industrial demand for cleaner chemical processes. The global de-aromatization agents market is expected to expand at a steady rate, driven by the growing emphasis on sustainable and eco-friendly manufacturing practices across various industries.
In the chemical processing sector, there is a rising need for effective de-aromatization solutions to remove aromatic compounds from various products and processes. This demand is particularly strong in industries such as petrochemicals, pharmaceuticals, and fine chemicals, where the presence of aromatic compounds can negatively impact product quality and environmental compliance.
Ethyl propanoate, as a potential de-aromatization agent, is gaining attention due to its favorable properties and performance characteristics. Its market potential is closely tied to its ability to effectively remove aromatic compounds while maintaining process efficiency and product quality. The increasing focus on green chemistry and sustainable manufacturing practices is expected to further boost the demand for ethyl propanoate and similar de-aromatization agents.
The Asia-Pacific region is anticipated to be a key growth market for de-aromatization agents, including ethyl propanoate. This is primarily due to the rapid industrialization in countries like China and India, coupled with stringent environmental regulations being implemented across the region. North America and Europe are also significant markets, driven by established chemical industries and strict environmental standards.
Key factors influencing the market demand for de-aromatization agents include regulatory compliance, cost-effectiveness, and performance efficiency. As industries strive to meet increasingly stringent environmental standards, the adoption of effective de-aromatization solutions becomes crucial. Ethyl propanoate's potential in this market will largely depend on its ability to offer superior performance compared to existing alternatives, as well as its cost-competitiveness and ease of integration into existing chemical processes.
The market for de-aromatization agents is characterized by ongoing research and development efforts aimed at improving efficiency and expanding application areas. This presents opportunities for ethyl propanoate to establish itself as a preferred solution in specific niches or applications where its unique properties offer distinct advantages over conventional de-aromatization methods.
In the chemical processing sector, there is a rising need for effective de-aromatization solutions to remove aromatic compounds from various products and processes. This demand is particularly strong in industries such as petrochemicals, pharmaceuticals, and fine chemicals, where the presence of aromatic compounds can negatively impact product quality and environmental compliance.
Ethyl propanoate, as a potential de-aromatization agent, is gaining attention due to its favorable properties and performance characteristics. Its market potential is closely tied to its ability to effectively remove aromatic compounds while maintaining process efficiency and product quality. The increasing focus on green chemistry and sustainable manufacturing practices is expected to further boost the demand for ethyl propanoate and similar de-aromatization agents.
The Asia-Pacific region is anticipated to be a key growth market for de-aromatization agents, including ethyl propanoate. This is primarily due to the rapid industrialization in countries like China and India, coupled with stringent environmental regulations being implemented across the region. North America and Europe are also significant markets, driven by established chemical industries and strict environmental standards.
Key factors influencing the market demand for de-aromatization agents include regulatory compliance, cost-effectiveness, and performance efficiency. As industries strive to meet increasingly stringent environmental standards, the adoption of effective de-aromatization solutions becomes crucial. Ethyl propanoate's potential in this market will largely depend on its ability to offer superior performance compared to existing alternatives, as well as its cost-competitiveness and ease of integration into existing chemical processes.
The market for de-aromatization agents is characterized by ongoing research and development efforts aimed at improving efficiency and expanding application areas. This presents opportunities for ethyl propanoate to establish itself as a preferred solution in specific niches or applications where its unique properties offer distinct advantages over conventional de-aromatization methods.
Current State and Challenges in De-Aromatization
De-aromatization processes in the chemical industry have gained significant attention due to their potential to reduce environmental impact and improve product quality. Currently, the state of de-aromatization technology is characterized by a mix of established methods and emerging innovations. Traditional approaches, such as hydrogenation and adsorption, remain widely used but face challenges in efficiency and selectivity.
The use of ethyl propanoate for de-aromatization represents a novel approach that is still in the early stages of research and development. This ester compound shows promise in selectively removing aromatic compounds from various chemical streams, potentially offering advantages over conventional methods. However, the technology is not yet fully mature and faces several challenges that need to be addressed before widespread industrial adoption.
One of the primary challenges in de-aromatization using ethyl propanoate is optimizing the reaction conditions to achieve high selectivity and conversion rates. Researchers are grappling with issues such as catalyst design, reaction kinetics, and process integration. The development of highly efficient and stable catalysts that can facilitate the de-aromatization process while minimizing side reactions is a key area of focus.
Another significant challenge lies in scaling up the process from laboratory experiments to industrial-scale operations. This transition involves overcoming engineering hurdles related to reactor design, heat and mass transfer limitations, and process control. Additionally, the economic viability of using ethyl propanoate on a large scale needs to be thoroughly assessed, considering factors such as raw material costs, energy requirements, and potential byproduct formation.
Environmental considerations also pose challenges in the development of ethyl propanoate-based de-aromatization processes. While the goal is to reduce the environmental impact of aromatic compounds, it is crucial to ensure that the de-aromatization process itself does not introduce new environmental concerns. This includes managing any waste streams generated during the process and minimizing the overall carbon footprint of the technology.
The regulatory landscape surrounding de-aromatization technologies is another factor that researchers and industry players must navigate. As environmental regulations become increasingly stringent, there is a growing need for de-aromatization processes that can meet or exceed these standards. This creates both challenges and opportunities for the development of ethyl propanoate-based solutions.
Lastly, the integration of ethyl propanoate de-aromatization technology into existing chemical processes presents its own set of challenges. Compatibility with current infrastructure, potential retrofitting requirements, and the need for specialized equipment are all factors that need to be carefully considered. The industry must also address the training and expertise required to operate and maintain these new de-aromatization systems effectively.
The use of ethyl propanoate for de-aromatization represents a novel approach that is still in the early stages of research and development. This ester compound shows promise in selectively removing aromatic compounds from various chemical streams, potentially offering advantages over conventional methods. However, the technology is not yet fully mature and faces several challenges that need to be addressed before widespread industrial adoption.
One of the primary challenges in de-aromatization using ethyl propanoate is optimizing the reaction conditions to achieve high selectivity and conversion rates. Researchers are grappling with issues such as catalyst design, reaction kinetics, and process integration. The development of highly efficient and stable catalysts that can facilitate the de-aromatization process while minimizing side reactions is a key area of focus.
Another significant challenge lies in scaling up the process from laboratory experiments to industrial-scale operations. This transition involves overcoming engineering hurdles related to reactor design, heat and mass transfer limitations, and process control. Additionally, the economic viability of using ethyl propanoate on a large scale needs to be thoroughly assessed, considering factors such as raw material costs, energy requirements, and potential byproduct formation.
Environmental considerations also pose challenges in the development of ethyl propanoate-based de-aromatization processes. While the goal is to reduce the environmental impact of aromatic compounds, it is crucial to ensure that the de-aromatization process itself does not introduce new environmental concerns. This includes managing any waste streams generated during the process and minimizing the overall carbon footprint of the technology.
The regulatory landscape surrounding de-aromatization technologies is another factor that researchers and industry players must navigate. As environmental regulations become increasingly stringent, there is a growing need for de-aromatization processes that can meet or exceed these standards. This creates both challenges and opportunities for the development of ethyl propanoate-based solutions.
Lastly, the integration of ethyl propanoate de-aromatization technology into existing chemical processes presents its own set of challenges. Compatibility with current infrastructure, potential retrofitting requirements, and the need for specialized equipment are all factors that need to be carefully considered. The industry must also address the training and expertise required to operate and maintain these new de-aromatization systems effectively.
Existing De-Aromatization Solutions
01 Catalytic hydrogenation for de-aromatization
Catalytic hydrogenation is a common method for de-aromatization of ethyl propanoate. This process involves using hydrogen gas and a suitable catalyst to saturate aromatic compounds, converting them to aliphatic structures. The choice of catalyst and reaction conditions are crucial for achieving efficient de-aromatization while maintaining the ester structure of ethyl propanoate.- Catalytic hydrogenation for de-aromatization: Catalytic hydrogenation is a common method for de-aromatization of ethyl propanoate. This process involves using a catalyst, typically a noble metal or transition metal, to facilitate the addition of hydrogen to aromatic compounds, converting them to saturated or partially saturated structures. The reaction conditions, such as temperature, pressure, and catalyst type, can be optimized to achieve efficient de-aromatization while preserving the ester functionality.
- Selective adsorption techniques: Selective adsorption techniques can be employed to remove aromatic compounds from ethyl propanoate. This method utilizes adsorbent materials with high affinity for aromatic structures, such as activated carbon, zeolites, or specialized polymeric resins. The adsorbents can be packed in columns or used in batch processes to selectively remove aromatic impurities from the ester, resulting in a de-aromatized product.
- Membrane separation processes: Membrane separation processes can be utilized for the de-aromatization of ethyl propanoate. This approach involves using specialized membranes that selectively permeate either the aromatic compounds or the ester, allowing for their separation. Various membrane types, such as polymeric or ceramic membranes, can be employed, and the process can be optimized by adjusting parameters like pressure, temperature, and membrane composition to achieve efficient de-aromatization.
- Oxidative de-aromatization: Oxidative de-aromatization can be applied to remove aromatic compounds from ethyl propanoate. This method involves using oxidizing agents or catalysts to convert aromatic structures into more polar compounds, which can then be separated from the ester. The oxidation process can be controlled to minimize the impact on the ester functionality while effectively removing aromatic impurities.
- Extractive distillation for de-aromatization: Extractive distillation can be employed to separate aromatic compounds from ethyl propanoate. This technique involves the addition of a solvent with high selectivity for aromatic compounds, which alters the relative volatility of the components in the mixture. The process allows for the separation of aromatic impurities from the ester through careful control of distillation conditions and solvent selection.
02 Selective adsorption techniques
Selective adsorption methods can be employed to remove aromatic compounds from ethyl propanoate. This approach utilizes adsorbent materials with high affinity for aromatic structures, allowing for their separation from the desired ester. The selection of appropriate adsorbents and optimization of process parameters are key factors in achieving effective de-aromatization.Expand Specific Solutions03 Membrane separation processes
Membrane-based separation techniques can be utilized for the de-aromatization of ethyl propanoate. These processes involve the use of selective membranes that allow the passage of the desired ester while retaining aromatic impurities. The development of specialized membrane materials and optimization of operating conditions are crucial for achieving high separation efficiency.Expand Specific Solutions04 Extractive distillation methods
Extractive distillation can be employed to separate aromatic compounds from ethyl propanoate. This process involves the addition of a solvent with high selectivity for aromatic structures, altering the relative volatility of the components and facilitating their separation. The selection of an appropriate solvent and optimization of distillation parameters are essential for effective de-aromatization.Expand Specific Solutions05 Oxidative de-aromatization techniques
Oxidative methods can be used for the de-aromatization of ethyl propanoate. These techniques involve the use of oxidizing agents to convert aromatic compounds into more easily separable products. The selection of suitable oxidizing agents and control of reaction conditions are crucial to prevent unwanted side reactions and maintain the integrity of the ethyl propanoate molecule.Expand Specific Solutions
Key Players in Chemical De-Aromatization Industry
The research on Ethyl Propanoate for de-aromatization in chemical processes is in a developing stage, with growing market potential due to increasing demand for eco-friendly solvents. The technology's maturity is moderate, with major players like Shell Oil Co., China Petroleum & Chemical Corp., and UBE Corp. actively involved in research and development. These companies are leveraging their expertise in petrochemicals and chemical processes to advance the technology. The competitive landscape is characterized by a mix of established oil and chemical companies, as well as specialized research institutes, indicating a diverse and dynamic field with opportunities for innovation and market growth.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to de-aromatization using ethyl propanoate. Their process involves a catalytic reaction where ethyl propanoate acts as both a solvent and a reactant. The method employs a proprietary zeolite catalyst that selectively removes aromatic compounds from petroleum fractions[1]. This technique has shown a de-aromatization efficiency of up to 95% in laboratory tests, with minimal impact on other desirable hydrocarbon components[3]. Sinopec has also integrated this process into their existing refinery operations, allowing for seamless adoption without significant infrastructure changes[5].
Strengths: High de-aromatization efficiency, integration with existing infrastructure, and minimal impact on desirable hydrocarbons. Weaknesses: Potential high costs associated with catalyst production and regeneration, and possible limitations in handling heavy aromatic compounds.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has pioneered a novel de-aromatization process utilizing ethyl propanoate in combination with advanced membrane technology. Their approach involves a two-step process: first, the aromatic-rich feedstock is treated with ethyl propanoate under controlled conditions, followed by a membrane separation step[2]. The membrane, developed by Shell's materials science team, selectively allows ethyl propanoate-aromatic complexes to permeate while retaining other hydrocarbons[4]. This process has demonstrated a de-aromatization rate of up to 98% in pilot plant trials, with the added benefit of easy aromatic recovery from the permeate stream[6]. Shell has also developed a proprietary catalyst system that enhances the selectivity of the ethyl propanoate-aromatic interaction, further improving process efficiency[8].
Strengths: Extremely high de-aromatization rate, easy aromatic recovery, and enhanced selectivity. Weaknesses: Potential high capital costs for membrane systems and the need for frequent membrane replacement.
Core Innovations in Ethyl Propanoate Application
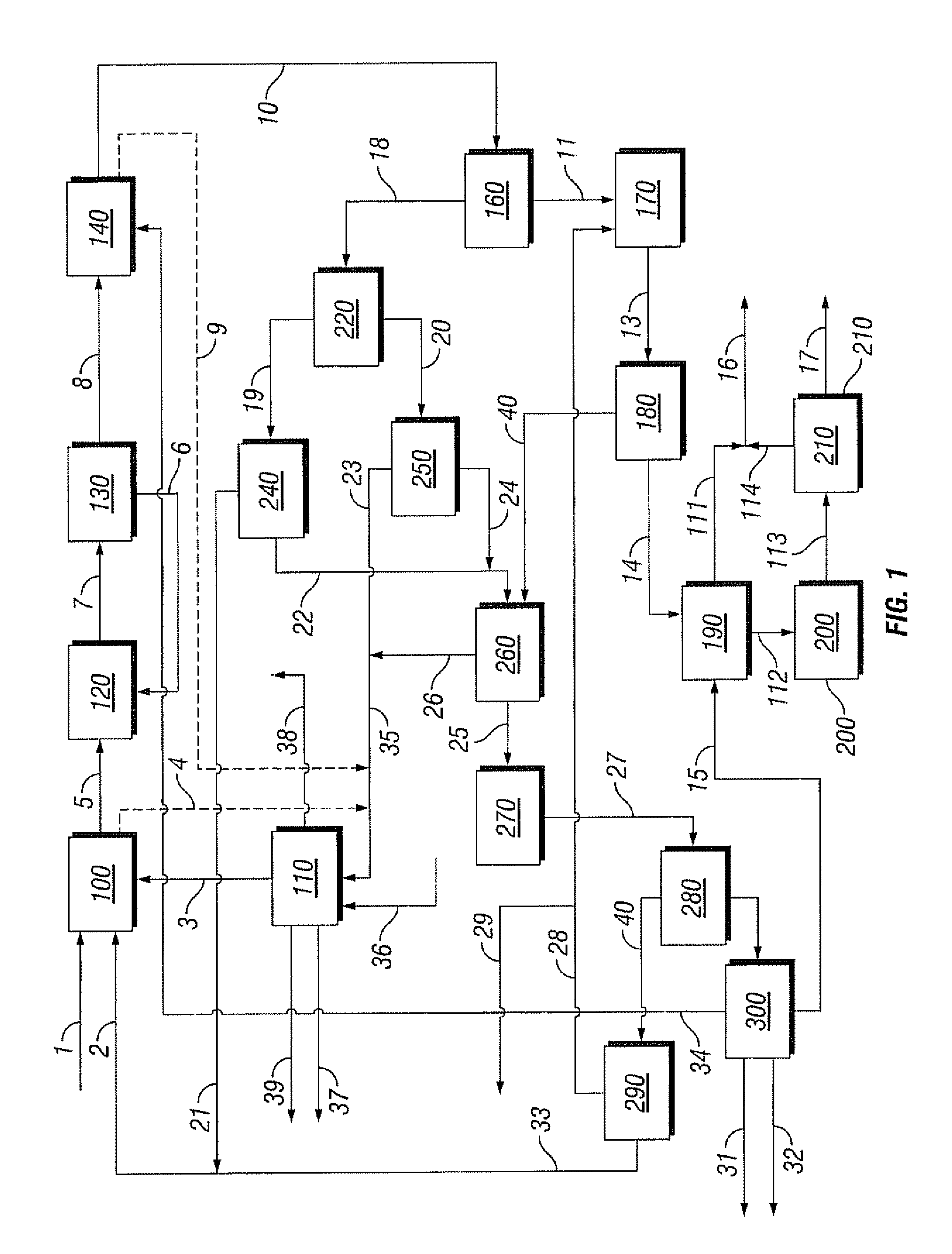
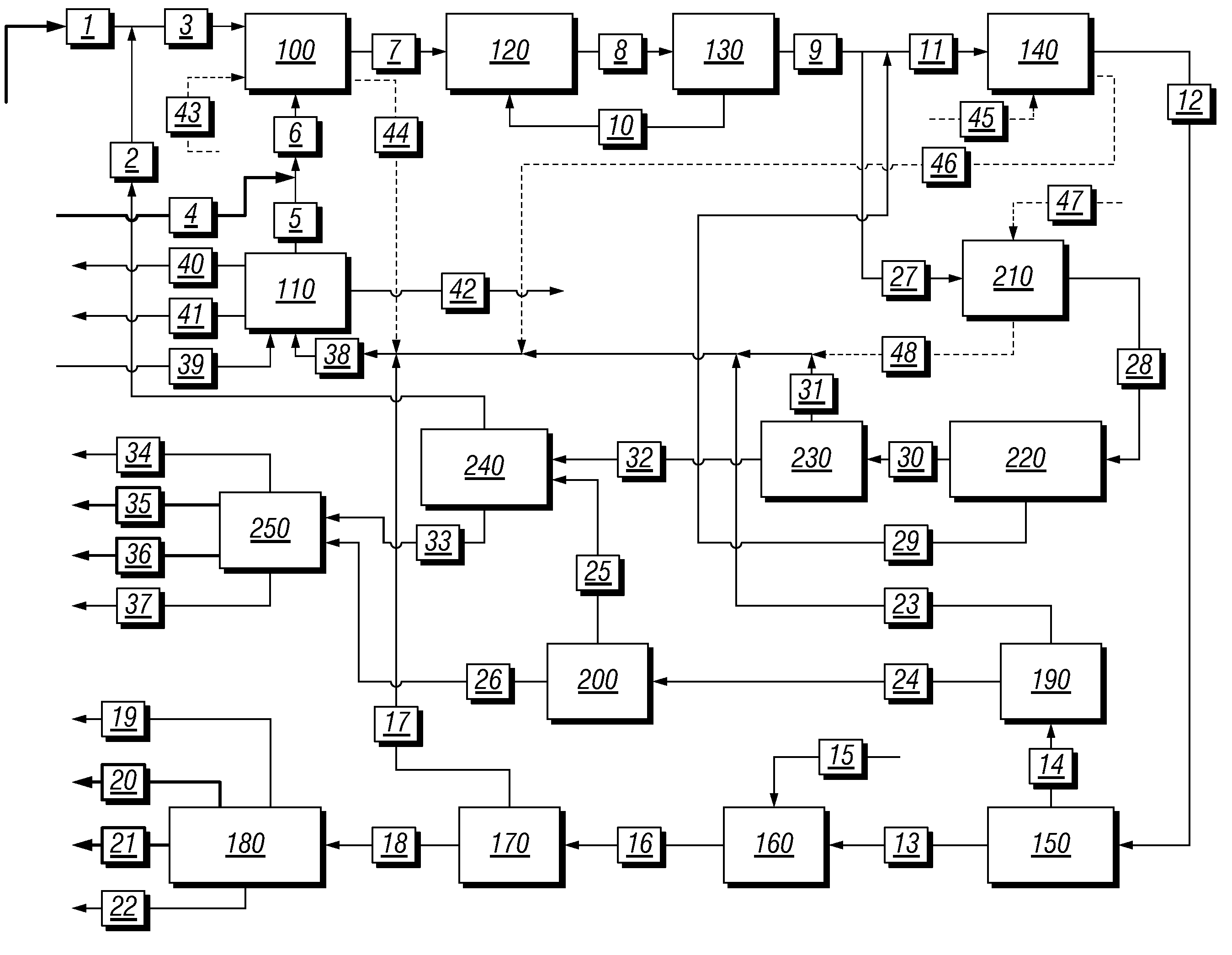
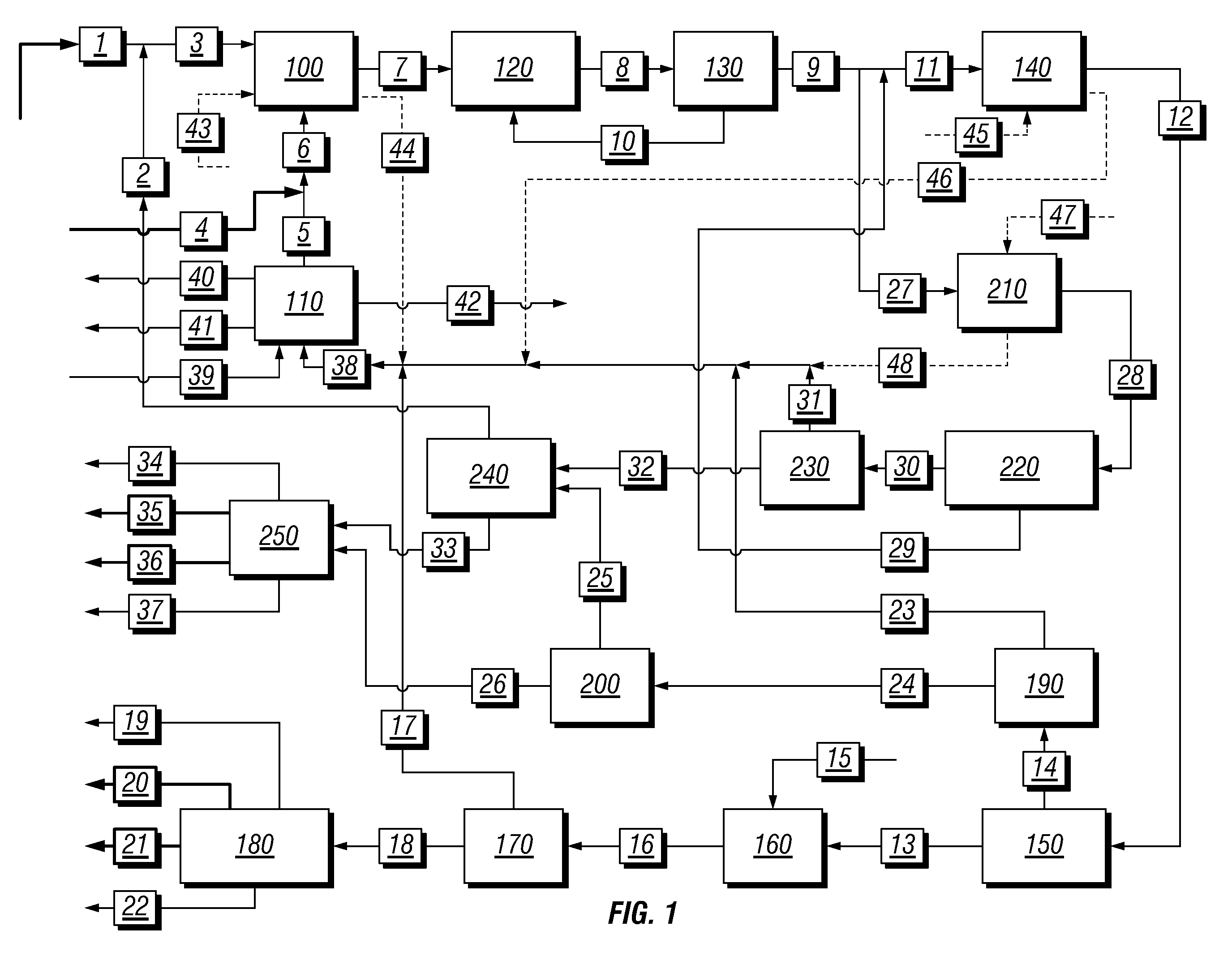
Integrated process to coproduce aromatic hydrocarbons and ethylene and propylene
PatentInactiveUS8017822B2
Innovation
- An integrated process involving the bromination of low molecular weight alkanes to produce monohaloalkanes, followed by coupling with a catalyst to form aromatic hydrocarbons and C2+ alkanes, with subsequent cracking to produce ethylene and propylene, while separating and recycling streams to optimize product yield and reduce unwanted byproducts.
Integrated process to coproduce aromatic hydrocarbons and ethylene and propylene
PatentInactiveUS20100234637A1
Innovation
- An integrated process that produces aromatic hydrocarbons and ethylene/propylene by contacting low molecular weight alkanes with bromine, separating monohaloalkanes, and using coupling catalysts to produce higher molecular weight hydrocarbons, while recycling streams to optimize product ratios and reduce unwanted byproducts.
Environmental Impact Assessment
The environmental impact assessment of ethyl propanoate for de-aromatization in chemical processes reveals both positive and negative aspects that warrant careful consideration. On the positive side, ethyl propanoate is generally considered less toxic and more environmentally friendly compared to many traditional aromatic solvents used in chemical processes. Its lower volatility and reduced emissions contribute to improved air quality and reduced atmospheric pollution.
However, the production and use of ethyl propanoate still have environmental implications that need to be addressed. The synthesis of ethyl propanoate typically involves the reaction of ethanol with propionic acid or its derivatives, which may require energy-intensive processes and potentially generate waste products. The environmental footprint of these production methods should be evaluated and optimized to minimize resource consumption and emissions.
In terms of aquatic ecosystems, ethyl propanoate has a relatively low water solubility and is biodegradable, which reduces its potential for long-term accumulation in water bodies. Nevertheless, accidental spills or improper disposal could still pose risks to aquatic life in the short term. Proper handling, storage, and disposal protocols must be implemented to mitigate these risks.
The use of ethyl propanoate in de-aromatization processes may lead to reduced overall environmental impact compared to traditional aromatic solvents. Its lower toxicity and improved biodegradability can result in decreased soil and groundwater contamination risks. However, the potential for soil contamination still exists, particularly in cases of large-scale industrial use or accidental releases.
From a lifecycle perspective, the environmental impact of ethyl propanoate should be assessed holistically, considering factors such as raw material sourcing, energy consumption during production, transportation, and end-of-life disposal or recycling. The potential for developing more sustainable production methods, such as using bio-based feedstocks or implementing green chemistry principles, should be explored to further reduce its environmental footprint.
Regulatory compliance and industry standards play a crucial role in managing the environmental impact of ethyl propanoate. Adherence to emissions regulations, waste management guidelines, and occupational safety standards is essential for minimizing negative environmental consequences. Additionally, ongoing monitoring and assessment of its long-term effects on ecosystems and human health should be conducted to ensure its continued safe use in chemical processes.
However, the production and use of ethyl propanoate still have environmental implications that need to be addressed. The synthesis of ethyl propanoate typically involves the reaction of ethanol with propionic acid or its derivatives, which may require energy-intensive processes and potentially generate waste products. The environmental footprint of these production methods should be evaluated and optimized to minimize resource consumption and emissions.
In terms of aquatic ecosystems, ethyl propanoate has a relatively low water solubility and is biodegradable, which reduces its potential for long-term accumulation in water bodies. Nevertheless, accidental spills or improper disposal could still pose risks to aquatic life in the short term. Proper handling, storage, and disposal protocols must be implemented to mitigate these risks.
The use of ethyl propanoate in de-aromatization processes may lead to reduced overall environmental impact compared to traditional aromatic solvents. Its lower toxicity and improved biodegradability can result in decreased soil and groundwater contamination risks. However, the potential for soil contamination still exists, particularly in cases of large-scale industrial use or accidental releases.
From a lifecycle perspective, the environmental impact of ethyl propanoate should be assessed holistically, considering factors such as raw material sourcing, energy consumption during production, transportation, and end-of-life disposal or recycling. The potential for developing more sustainable production methods, such as using bio-based feedstocks or implementing green chemistry principles, should be explored to further reduce its environmental footprint.
Regulatory compliance and industry standards play a crucial role in managing the environmental impact of ethyl propanoate. Adherence to emissions regulations, waste management guidelines, and occupational safety standards is essential for minimizing negative environmental consequences. Additionally, ongoing monitoring and assessment of its long-term effects on ecosystems and human health should be conducted to ensure its continued safe use in chemical processes.
Regulatory Compliance for Chemical Processes
Regulatory compliance is a critical aspect of chemical processes involving ethyl propanoate for de-aromatization. The use of this compound in industrial applications is subject to various regulations and standards set by governmental agencies and international organizations.
In the United States, the Environmental Protection Agency (EPA) regulates the use of ethyl propanoate under the Toxic Substances Control Act (TSCA). Manufacturers and importers must comply with reporting, record-keeping, and testing requirements. The Occupational Safety and Health Administration (OSHA) also sets standards for workplace safety, including exposure limits and handling procedures for ethyl propanoate.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation applies to ethyl propanoate used in de-aromatization processes. Companies must register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets to downstream users.
Globally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Ethyl propanoate must be labeled and packaged according to GHS guidelines, including appropriate hazard pictograms, signal words, and safety precautions.
Transportation of ethyl propanoate is regulated by international agreements such as the International Maritime Dangerous Goods (IMDG) Code for sea transport and the International Air Transport Association (IATA) Dangerous Goods Regulations for air transport. These regulations specify packaging, labeling, and documentation requirements for safe transportation.
Waste management regulations also apply to the disposal of ethyl propanoate and its by-products. The Resource Conservation and Recovery Act (RCRA) in the US and the Waste Framework Directive in the EU provide guidelines for proper handling and disposal of chemical waste.
Companies using ethyl propanoate in de-aromatization processes must implement robust environmental management systems to ensure compliance with air and water quality regulations. This may include obtaining permits for emissions and discharges, as well as implementing monitoring and reporting protocols.
To maintain regulatory compliance, organizations should establish comprehensive training programs for employees handling ethyl propanoate. These programs should cover safe handling procedures, emergency response protocols, and proper use of personal protective equipment.
Regular audits and inspections are essential to verify compliance with all applicable regulations. Companies should maintain detailed records of their chemical inventory, usage, and disposal practices to demonstrate adherence to regulatory requirements during inspections or in case of incidents.
In the United States, the Environmental Protection Agency (EPA) regulates the use of ethyl propanoate under the Toxic Substances Control Act (TSCA). Manufacturers and importers must comply with reporting, record-keeping, and testing requirements. The Occupational Safety and Health Administration (OSHA) also sets standards for workplace safety, including exposure limits and handling procedures for ethyl propanoate.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation applies to ethyl propanoate used in de-aromatization processes. Companies must register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets to downstream users.
Globally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Ethyl propanoate must be labeled and packaged according to GHS guidelines, including appropriate hazard pictograms, signal words, and safety precautions.
Transportation of ethyl propanoate is regulated by international agreements such as the International Maritime Dangerous Goods (IMDG) Code for sea transport and the International Air Transport Association (IATA) Dangerous Goods Regulations for air transport. These regulations specify packaging, labeling, and documentation requirements for safe transportation.
Waste management regulations also apply to the disposal of ethyl propanoate and its by-products. The Resource Conservation and Recovery Act (RCRA) in the US and the Waste Framework Directive in the EU provide guidelines for proper handling and disposal of chemical waste.
Companies using ethyl propanoate in de-aromatization processes must implement robust environmental management systems to ensure compliance with air and water quality regulations. This may include obtaining permits for emissions and discharges, as well as implementing monitoring and reporting protocols.
To maintain regulatory compliance, organizations should establish comprehensive training programs for employees handling ethyl propanoate. These programs should cover safe handling procedures, emergency response protocols, and proper use of personal protective equipment.
Regular audits and inspections are essential to verify compliance with all applicable regulations. Companies should maintain detailed records of their chemical inventory, usage, and disposal practices to demonstrate adherence to regulatory requirements during inspections or in case of incidents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!