Ethyl Propanoate in Biochemical Pathway Regulation Studies
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Background and Research Objectives
Ethyl propanoate, also known as ethyl propionate, is a naturally occurring ester compound found in various fruits and fermented products. Its chemical formula is C5H10O2, and it is characterized by a sweet, fruity odor reminiscent of pineapples. This compound has gained significant attention in biochemical pathway regulation studies due to its potential role in cellular metabolism and signaling processes.
The research on ethyl propanoate in biochemical pathway regulation has evolved over the past few decades, driven by advancements in analytical techniques and a growing understanding of cellular metabolic networks. Initially, studies focused on its presence in natural sources and its role as a flavor compound. However, recent investigations have expanded to explore its potential as a metabolic regulator and signaling molecule.
The primary objective of research in this field is to elucidate the mechanisms by which ethyl propanoate influences biochemical pathways and cellular functions. This includes understanding its interactions with enzymes, receptors, and other cellular components that may be involved in metabolic regulation. Additionally, researchers aim to identify specific pathways that are modulated by ethyl propanoate and determine the physiological consequences of these interactions.
One key area of interest is the potential role of ethyl propanoate in energy metabolism. Some studies suggest that this compound may influence pathways related to glucose utilization, lipid metabolism, and mitochondrial function. Researchers are investigating whether ethyl propanoate acts as a direct metabolic substrate, a signaling molecule, or both in these processes.
Another important aspect of the research is the exploration of ethyl propanoate's effects on gene expression and protein synthesis. This involves studying its impact on transcription factors, epigenetic modifications, and post-translational protein modifications that may contribute to pathway regulation.
The potential therapeutic applications of ethyl propanoate are also driving research efforts. Scientists are investigating its possible use in treating metabolic disorders, inflammatory conditions, and certain types of cancer. Understanding the compound's role in biochemical pathway regulation could lead to the development of novel therapeutic strategies targeting specific metabolic pathways.
As research progresses, there is a growing interest in developing synthetic analogs of ethyl propanoate with enhanced regulatory properties. These efforts aim to create more potent or selective compounds that can be used as research tools or potential therapeutic agents.
The ultimate goal of this research is to gain a comprehensive understanding of ethyl propanoate's role in cellular metabolism and signaling, potentially leading to new insights into fundamental biological processes and innovative approaches to disease treatment. This multifaceted research endeavor combines elements of biochemistry, molecular biology, and systems biology to unravel the complex interactions between ethyl propanoate and cellular regulatory networks.
The research on ethyl propanoate in biochemical pathway regulation has evolved over the past few decades, driven by advancements in analytical techniques and a growing understanding of cellular metabolic networks. Initially, studies focused on its presence in natural sources and its role as a flavor compound. However, recent investigations have expanded to explore its potential as a metabolic regulator and signaling molecule.
The primary objective of research in this field is to elucidate the mechanisms by which ethyl propanoate influences biochemical pathways and cellular functions. This includes understanding its interactions with enzymes, receptors, and other cellular components that may be involved in metabolic regulation. Additionally, researchers aim to identify specific pathways that are modulated by ethyl propanoate and determine the physiological consequences of these interactions.
One key area of interest is the potential role of ethyl propanoate in energy metabolism. Some studies suggest that this compound may influence pathways related to glucose utilization, lipid metabolism, and mitochondrial function. Researchers are investigating whether ethyl propanoate acts as a direct metabolic substrate, a signaling molecule, or both in these processes.
Another important aspect of the research is the exploration of ethyl propanoate's effects on gene expression and protein synthesis. This involves studying its impact on transcription factors, epigenetic modifications, and post-translational protein modifications that may contribute to pathway regulation.
The potential therapeutic applications of ethyl propanoate are also driving research efforts. Scientists are investigating its possible use in treating metabolic disorders, inflammatory conditions, and certain types of cancer. Understanding the compound's role in biochemical pathway regulation could lead to the development of novel therapeutic strategies targeting specific metabolic pathways.
As research progresses, there is a growing interest in developing synthetic analogs of ethyl propanoate with enhanced regulatory properties. These efforts aim to create more potent or selective compounds that can be used as research tools or potential therapeutic agents.
The ultimate goal of this research is to gain a comprehensive understanding of ethyl propanoate's role in cellular metabolism and signaling, potentially leading to new insights into fundamental biological processes and innovative approaches to disease treatment. This multifaceted research endeavor combines elements of biochemistry, molecular biology, and systems biology to unravel the complex interactions between ethyl propanoate and cellular regulatory networks.
Market Analysis for Ethyl Propanoate in Biochemical Research
The market for ethyl propanoate in biochemical research is experiencing significant growth, driven by its increasing applications in pathway regulation studies. This compound, also known as ethyl propionate, plays a crucial role in understanding and manipulating biochemical pathways, making it a valuable tool for researchers in various fields of life sciences.
The demand for ethyl propanoate is primarily fueled by the expanding biotechnology and pharmaceutical industries. As these sectors continue to invest heavily in research and development, the need for reliable and effective compounds for pathway regulation studies has surged. Ethyl propanoate's unique properties make it particularly useful in investigating cellular processes, enzyme kinetics, and metabolic pathways.
In the pharmaceutical industry, ethyl propanoate is utilized in drug discovery and development processes. It serves as a model compound for studying esterase activity and drug metabolism, which are critical aspects of pharmacokinetics. This application has led to a steady increase in demand from pharmaceutical research laboratories and contract research organizations (CROs).
The academic research sector also contributes significantly to the market demand for ethyl propanoate. Universities and research institutions conducting fundamental biochemical research rely on this compound for various experimental protocols. Its use in studying lipid metabolism, cellular signaling, and gene expression regulation has made it an essential reagent in many laboratories.
The global market for biochemical research reagents, including ethyl propanoate, is projected to grow steadily over the next five years. This growth is attributed to increased funding for life sciences research, advancements in biotechnology, and the rising prevalence of chronic diseases driving pharmaceutical research.
Geographically, North America and Europe dominate the market for ethyl propanoate in biochemical research, owing to their well-established research infrastructure and high concentration of biotechnology and pharmaceutical companies. However, the Asia-Pacific region is expected to witness the fastest growth in demand, driven by increasing investments in life sciences research and the expansion of the biotechnology sector in countries like China and India.
The market for ethyl propanoate is characterized by a mix of large chemical manufacturers and specialized biochemical suppliers. Key players in this market focus on product purity, consistency, and customization to meet the specific requirements of research applications. As the field of biochemical pathway regulation continues to evolve, suppliers are likely to develop new formulations and grades of ethyl propanoate tailored to emerging research needs.
The demand for ethyl propanoate is primarily fueled by the expanding biotechnology and pharmaceutical industries. As these sectors continue to invest heavily in research and development, the need for reliable and effective compounds for pathway regulation studies has surged. Ethyl propanoate's unique properties make it particularly useful in investigating cellular processes, enzyme kinetics, and metabolic pathways.
In the pharmaceutical industry, ethyl propanoate is utilized in drug discovery and development processes. It serves as a model compound for studying esterase activity and drug metabolism, which are critical aspects of pharmacokinetics. This application has led to a steady increase in demand from pharmaceutical research laboratories and contract research organizations (CROs).
The academic research sector also contributes significantly to the market demand for ethyl propanoate. Universities and research institutions conducting fundamental biochemical research rely on this compound for various experimental protocols. Its use in studying lipid metabolism, cellular signaling, and gene expression regulation has made it an essential reagent in many laboratories.
The global market for biochemical research reagents, including ethyl propanoate, is projected to grow steadily over the next five years. This growth is attributed to increased funding for life sciences research, advancements in biotechnology, and the rising prevalence of chronic diseases driving pharmaceutical research.
Geographically, North America and Europe dominate the market for ethyl propanoate in biochemical research, owing to their well-established research infrastructure and high concentration of biotechnology and pharmaceutical companies. However, the Asia-Pacific region is expected to witness the fastest growth in demand, driven by increasing investments in life sciences research and the expansion of the biotechnology sector in countries like China and India.
The market for ethyl propanoate is characterized by a mix of large chemical manufacturers and specialized biochemical suppliers. Key players in this market focus on product purity, consistency, and customization to meet the specific requirements of research applications. As the field of biochemical pathway regulation continues to evolve, suppliers are likely to develop new formulations and grades of ethyl propanoate tailored to emerging research needs.
Current Challenges in Biochemical Pathway Regulation
The field of biochemical pathway regulation faces several significant challenges, particularly in the context of research on ethyl propanoate. One of the primary obstacles is the complexity of metabolic networks, which makes it difficult to predict and control the effects of manipulating specific pathways. Ethyl propanoate, as a model compound, exemplifies this challenge due to its involvement in multiple interconnected metabolic routes.
Researchers struggle with the precise control of enzyme activity and substrate specificity when attempting to regulate pathways involving ethyl propanoate. The dynamic nature of cellular metabolism often leads to unexpected compensatory mechanisms that can counteract intended regulatory effects. This unpredictability hampers efforts to optimize production or utilization of ethyl propanoate in biotechnological applications.
Another significant challenge lies in the development of robust and sensitive analytical methods for real-time monitoring of metabolic fluxes. Current techniques often lack the temporal and spatial resolution necessary to capture the rapid changes in metabolite concentrations, including ethyl propanoate, within living cells. This limitation hinders the ability to fine-tune regulatory strategies and assess their immediate impacts on biochemical pathways.
The integration of multi-omics data presents another hurdle in pathway regulation studies. While high-throughput technologies generate vast amounts of data on gene expression, protein levels, and metabolite concentrations, interpreting this information in the context of ethyl propanoate metabolism remains challenging. Researchers struggle to develop comprehensive models that can accurately predict the outcomes of regulatory interventions across different cellular conditions and genetic backgrounds.
Environmental factors and cellular heterogeneity further complicate pathway regulation efforts. The behavior of biochemical pathways, including those involving ethyl propanoate, can vary significantly depending on factors such as pH, temperature, and nutrient availability. Additionally, cell-to-cell variability within populations can lead to inconsistent responses to regulatory strategies, making it difficult to achieve uniform control across entire cultures or tissues.
Lastly, the translation of laboratory findings to industrial-scale applications poses a significant challenge. Regulatory mechanisms that work effectively in controlled experimental settings may not scale up efficiently or may encounter unforeseen obstacles when implemented in large-scale bioreactors or in vivo systems. This gap between fundamental research and practical application remains a critical bottleneck in the field of biochemical pathway regulation, particularly for compounds like ethyl propanoate with potential industrial significance.
Researchers struggle with the precise control of enzyme activity and substrate specificity when attempting to regulate pathways involving ethyl propanoate. The dynamic nature of cellular metabolism often leads to unexpected compensatory mechanisms that can counteract intended regulatory effects. This unpredictability hampers efforts to optimize production or utilization of ethyl propanoate in biotechnological applications.
Another significant challenge lies in the development of robust and sensitive analytical methods for real-time monitoring of metabolic fluxes. Current techniques often lack the temporal and spatial resolution necessary to capture the rapid changes in metabolite concentrations, including ethyl propanoate, within living cells. This limitation hinders the ability to fine-tune regulatory strategies and assess their immediate impacts on biochemical pathways.
The integration of multi-omics data presents another hurdle in pathway regulation studies. While high-throughput technologies generate vast amounts of data on gene expression, protein levels, and metabolite concentrations, interpreting this information in the context of ethyl propanoate metabolism remains challenging. Researchers struggle to develop comprehensive models that can accurately predict the outcomes of regulatory interventions across different cellular conditions and genetic backgrounds.
Environmental factors and cellular heterogeneity further complicate pathway regulation efforts. The behavior of biochemical pathways, including those involving ethyl propanoate, can vary significantly depending on factors such as pH, temperature, and nutrient availability. Additionally, cell-to-cell variability within populations can lead to inconsistent responses to regulatory strategies, making it difficult to achieve uniform control across entire cultures or tissues.
Lastly, the translation of laboratory findings to industrial-scale applications poses a significant challenge. Regulatory mechanisms that work effectively in controlled experimental settings may not scale up efficiently or may encounter unforeseen obstacles when implemented in large-scale bioreactors or in vivo systems. This gap between fundamental research and practical application remains a critical bottleneck in the field of biochemical pathway regulation, particularly for compounds like ethyl propanoate with potential industrial significance.
Existing Applications of Ethyl Propanoate
01 Synthesis of ethyl propanoate
Ethyl propanoate can be synthesized through various methods, including the esterification of propionic acid with ethanol. This process typically involves the use of catalysts and specific reaction conditions to optimize yield and purity.- Synthesis of ethyl propanoate: Ethyl propanoate can be synthesized through various methods, including esterification of propionic acid with ethanol, or by the reaction of ethyl alcohol with propionyl chloride. These processes often involve catalysts and specific reaction conditions to optimize yield and purity.
- Applications in fragrance and flavor industry: Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is often incorporated into artificial fruit flavors, particularly for pineapple and strawberry scents. The compound is also used in perfumery to create various fragrance compositions.
- Use as a solvent and intermediate: Ethyl propanoate serves as an important solvent in various industrial applications, including paints, inks, and coatings. It is also used as an intermediate in the production of other chemicals and pharmaceuticals, owing to its reactivity and versatility in organic synthesis.
- Production methods and process improvements: Various methods and process improvements have been developed for the industrial production of ethyl propanoate. These include continuous flow reactors, improved catalysts, and optimized reaction conditions to enhance yield, reduce waste, and increase energy efficiency in the manufacturing process.
- Environmental and safety considerations: As ethyl propanoate is used in various consumer products and industrial processes, there is ongoing research into its environmental impact and safety profile. This includes studies on biodegradability, toxicity, and the development of more environmentally friendly production methods and applications.
02 Applications in fragrance and flavor industry
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is employed in the creation of artificial fruit flavors, particularly for pineapple and strawberry scents, and in various perfume formulations.Expand Specific Solutions03 Use in pharmaceutical formulations
Ethyl propanoate finds applications in pharmaceutical formulations, particularly as a solvent or excipient. It may be used in the preparation of various drug delivery systems or as a component in topical formulations.Expand Specific Solutions04 Industrial solvent and chemical intermediate
Ethyl propanoate serves as an industrial solvent and chemical intermediate in various processes. It is used in the production of other chemicals, paints, and coatings, and as a solvent in the electronics industry for cleaning applications.Expand Specific Solutions05 Environmental and safety considerations
The use and handling of ethyl propanoate require consideration of environmental and safety aspects. This includes proper storage, handling procedures, and disposal methods to minimize environmental impact and ensure worker safety in industrial settings.Expand Specific Solutions
Key Players in Biochemical Pathway Regulation Studies
The research on Ethyl Propanoate in biochemical pathway regulation studies is in a developing stage, with the market showing potential for growth. The technology is advancing, but still requires further refinement. Key players in this field include Yissum Research Development Co. Ltd., Genomatica, Inc., and Novozymes A/S, each contributing to the advancement of the technology. The market size is expanding as the applications of Ethyl Propanoate in biochemical pathways become more apparent. However, the technology's maturity level varies among companies, with some like Novozymes A/S and Genomatica, Inc. demonstrating more advanced capabilities in enzyme and bio-based process technologies, which could be applied to this research area.
Genomatica, Inc.
Technical Solution: Genomatica has developed a proprietary bioengineering platform for the production of ethyl propanoate through biochemical pathways. Their approach involves engineering microorganisms to produce ethyl propanoate from renewable feedstocks. The company has optimized fermentation processes to achieve high yields and purity of ethyl propanoate. They have also implemented advanced metabolic engineering techniques to enhance the efficiency of the biochemical pathways involved in ethyl propanoate synthesis. Genomatica's research has focused on identifying and manipulating key enzymes in the propanoate biosynthesis pathway to increase production rates[1]. Additionally, they have developed novel separation and purification methods to isolate ethyl propanoate from fermentation broths with high efficiency[2].
Strengths: Sustainable production from renewable resources, scalable fermentation process, high product purity. Weaknesses: Potentially higher production costs compared to petrochemical routes, limited by feedstock availability.
ZuvaSyntha Ltd.
Technical Solution: ZuvaSyntha has pioneered a novel approach to ethyl propanoate production using synthetic biology techniques. Their research focuses on designing artificial biochemical pathways for efficient ethyl propanoate synthesis. The company has engineered synthetic microorganisms with optimized metabolic pathways specifically tailored for ethyl propanoate production. ZuvaSyntha's technology incorporates advanced gene editing tools like CRISPR-Cas9 to fine-tune enzyme expression levels and pathway regulation[3]. They have also developed proprietary bioinformatics algorithms to predict and optimize metabolic flux for maximum ethyl propanoate yield. The company's research extends to exploring non-conventional microbial hosts that offer unique advantages for ethyl propanoate biosynthesis[4].
Strengths: Highly optimized and tailored biosynthetic pathways, potential for high yields and selectivity. Weaknesses: Regulatory challenges associated with genetically engineered organisms, potential scalability issues.
Core Innovations in Ethyl Propanoate Research
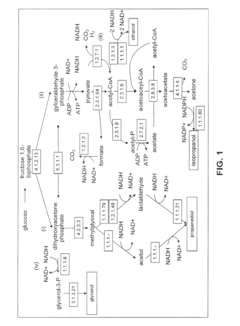
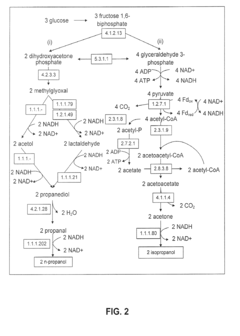
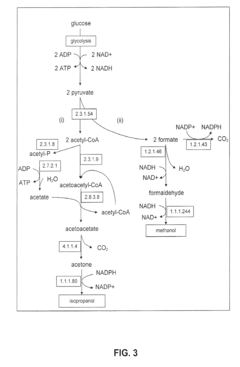
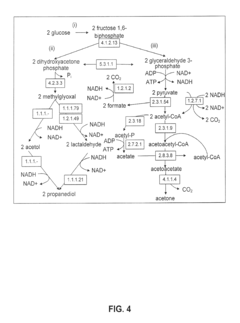
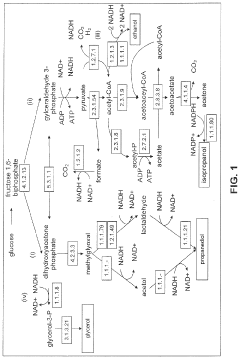
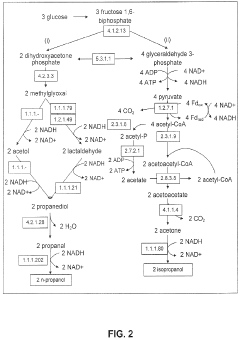
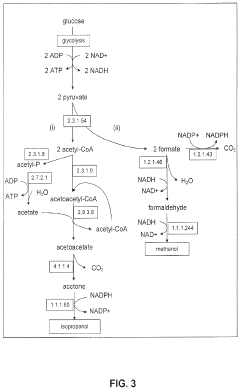
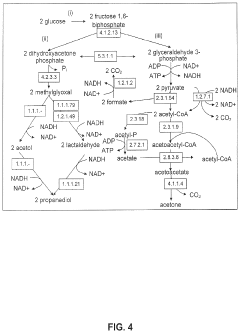
Production of Propanols, Alcohols, and Polyols in Consolidated Bioprocessing Organisms
PatentActiveUS20120322078A1
Innovation
- A consolidated bioprocessing (CBP) system that uses recombinant microorganisms to express native and heterologous enzymes for novel metabolic pathways, converting carbohydrates into valuable products like 1,2-propanediol, isopropanol, ethanol, glycerol, n-propanol, and acetone, thereby bypassing the need for dedicated cellulase production and reducing capital costs.
Production of propanols, alcohols, and polyols in consolidated bioprocessing organisms
PatentActiveUS20200325500A1
Innovation
- A consolidated bioprocessing (CBP) system that uses recombinant microorganisms to express native and heterologous enzymes for novel metabolic pathways, enabling efficient conversion of lignocellulosic biomass into products like 1,2-propanediol, isopropanol, ethanol, and glycerol, thereby reducing the need for external cellulases and lowering production costs.
Safety and Toxicology Considerations
Safety and toxicology considerations are paramount when researching ethyl propanoate in biochemical pathway regulation studies. This compound, while widely used in various industries, requires careful handling and assessment of potential risks to ensure the safety of researchers and the environment.
Ethyl propanoate, also known as ethyl propionate, is generally considered to have low toxicity. However, like many organic compounds, it can pose health risks if not handled properly. Inhalation of vapors may cause respiratory irritation, while skin contact can lead to mild irritation. Prolonged exposure to high concentrations may result in more severe effects, including dizziness, headaches, and nausea.
In laboratory settings, proper ventilation is crucial when working with ethyl propanoate. Researchers should use fume hoods and wear appropriate personal protective equipment, including gloves, lab coats, and safety goggles. It is essential to avoid skin contact and inhalation of vapors, particularly during the preparation and handling of solutions containing this compound.
From an environmental perspective, ethyl propanoate is biodegradable and does not persist in the environment for extended periods. However, care must be taken to prevent large-scale releases, as it can be harmful to aquatic life in high concentrations. Proper disposal methods should be employed, following local regulations and guidelines for chemical waste management.
When conducting biochemical pathway regulation studies, researchers must consider the potential interactions between ethyl propanoate and other cellular components. While the compound itself has low toxicity, its effects on specific biochemical pathways and cellular processes need to be carefully evaluated. This includes assessing potential off-target effects and unintended consequences on cellular metabolism.
Long-term toxicological studies on ethyl propanoate are limited, particularly in the context of its use in biochemical pathway regulation. As research in this area progresses, it is crucial to conduct comprehensive toxicological assessments, including genotoxicity, carcinogenicity, and reproductive toxicity studies, to ensure the compound's safety for prolonged use in biological systems.
In conclusion, while ethyl propanoate presents relatively low safety risks, proper precautions and thorough toxicological evaluations are essential when using it in biochemical pathway regulation studies. Researchers must adhere to established safety protocols, conduct ongoing risk assessments, and stay informed about any new findings regarding the compound's toxicological profile to ensure the safety and integrity of their research.
Ethyl propanoate, also known as ethyl propionate, is generally considered to have low toxicity. However, like many organic compounds, it can pose health risks if not handled properly. Inhalation of vapors may cause respiratory irritation, while skin contact can lead to mild irritation. Prolonged exposure to high concentrations may result in more severe effects, including dizziness, headaches, and nausea.
In laboratory settings, proper ventilation is crucial when working with ethyl propanoate. Researchers should use fume hoods and wear appropriate personal protective equipment, including gloves, lab coats, and safety goggles. It is essential to avoid skin contact and inhalation of vapors, particularly during the preparation and handling of solutions containing this compound.
From an environmental perspective, ethyl propanoate is biodegradable and does not persist in the environment for extended periods. However, care must be taken to prevent large-scale releases, as it can be harmful to aquatic life in high concentrations. Proper disposal methods should be employed, following local regulations and guidelines for chemical waste management.
When conducting biochemical pathway regulation studies, researchers must consider the potential interactions between ethyl propanoate and other cellular components. While the compound itself has low toxicity, its effects on specific biochemical pathways and cellular processes need to be carefully evaluated. This includes assessing potential off-target effects and unintended consequences on cellular metabolism.
Long-term toxicological studies on ethyl propanoate are limited, particularly in the context of its use in biochemical pathway regulation. As research in this area progresses, it is crucial to conduct comprehensive toxicological assessments, including genotoxicity, carcinogenicity, and reproductive toxicity studies, to ensure the compound's safety for prolonged use in biological systems.
In conclusion, while ethyl propanoate presents relatively low safety risks, proper precautions and thorough toxicological evaluations are essential when using it in biochemical pathway regulation studies. Researchers must adhere to established safety protocols, conduct ongoing risk assessments, and stay informed about any new findings regarding the compound's toxicological profile to ensure the safety and integrity of their research.
Ethical Implications in Biochemical Research
The ethical implications of research on ethyl propanoate in biochemical pathway regulation studies are multifaceted and require careful consideration. As this compound is widely used in the food industry as a flavoring agent, its study in biochemical contexts raises questions about potential impacts on human health and the environment.
One primary ethical concern is the responsible use of ethyl propanoate in research settings. Scientists must ensure that proper safety protocols are in place to protect laboratory personnel and prevent accidental release into the environment. This includes implementing appropriate handling procedures, waste disposal methods, and containment measures.
The potential for dual-use applications of ethyl propanoate research also presents ethical challenges. While the primary intent may be to understand biochemical pathways, findings could potentially be misused for harmful purposes, such as developing chemical weapons or manipulating biological systems in unintended ways. Researchers must be vigilant in considering the potential consequences of their work and implement safeguards against misuse.
Transparency and data sharing are crucial ethical considerations in this field. Researchers should strive to publish their findings openly and completely, allowing for peer review and replication of results. However, this must be balanced with the need to protect intellectual property rights and prevent the misuse of sensitive information.
The use of animal models in ethyl propanoate studies raises additional ethical questions. Researchers must justify the necessity of animal experiments and ensure that they adhere to established guidelines for humane treatment and minimization of suffering. Alternative methods, such as in vitro studies or computer simulations, should be explored whenever possible.
Ethical concerns also extend to the potential environmental impacts of increased ethyl propanoate production or use resulting from research findings. Scientists must consider the long-term ecological consequences of their work and strive to develop sustainable practices that minimize environmental harm.
Furthermore, the ethical implications of altering biochemical pathways through the use of ethyl propanoate or related compounds must be carefully evaluated. This includes considering potential unintended consequences on cellular function, organism health, and ecosystem balance. Researchers should adopt a precautionary approach and conduct thorough risk assessments before implementing any interventions based on their findings.
One primary ethical concern is the responsible use of ethyl propanoate in research settings. Scientists must ensure that proper safety protocols are in place to protect laboratory personnel and prevent accidental release into the environment. This includes implementing appropriate handling procedures, waste disposal methods, and containment measures.
The potential for dual-use applications of ethyl propanoate research also presents ethical challenges. While the primary intent may be to understand biochemical pathways, findings could potentially be misused for harmful purposes, such as developing chemical weapons or manipulating biological systems in unintended ways. Researchers must be vigilant in considering the potential consequences of their work and implement safeguards against misuse.
Transparency and data sharing are crucial ethical considerations in this field. Researchers should strive to publish their findings openly and completely, allowing for peer review and replication of results. However, this must be balanced with the need to protect intellectual property rights and prevent the misuse of sensitive information.
The use of animal models in ethyl propanoate studies raises additional ethical questions. Researchers must justify the necessity of animal experiments and ensure that they adhere to established guidelines for humane treatment and minimization of suffering. Alternative methods, such as in vitro studies or computer simulations, should be explored whenever possible.
Ethical concerns also extend to the potential environmental impacts of increased ethyl propanoate production or use resulting from research findings. Scientists must consider the long-term ecological consequences of their work and strive to develop sustainable practices that minimize environmental harm.
Furthermore, the ethical implications of altering biochemical pathways through the use of ethyl propanoate or related compounds must be carefully evaluated. This includes considering potential unintended consequences on cellular function, organism health, and ecosystem balance. Researchers should adopt a precautionary approach and conduct thorough risk assessments before implementing any interventions based on their findings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!