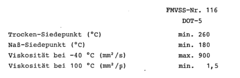Optimization of Ethyl Propanoate-Ester Brake Fluid Composition
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Brake Fluid Evolution
The evolution of brake fluid has been a critical aspect of automotive safety and performance improvement over the decades. Initially, in the early 20th century, brake systems relied on simple mineral oils or castor oil-based fluids. However, these early fluids had significant limitations, including low boiling points and poor resistance to moisture absorption, which led to brake failure under extreme conditions.
The 1930s saw the introduction of glycol-based brake fluids, marking a significant advancement in brake system technology. These fluids offered improved thermal stability and higher boiling points, addressing many of the shortcomings of earlier formulations. The development of polyglycol-based fluids in the 1950s further enhanced brake fluid performance, particularly in terms of moisture resistance and compatibility with rubber components in the brake system.
As automotive technology progressed, so did the demands on brake fluid performance. The 1960s and 1970s witnessed the introduction of silicone-based brake fluids, which offered exceptional thermal stability and resistance to moisture absorption. However, their higher compressibility and incompatibility with conventional brake systems limited their widespread adoption.
The late 20th century brought about a focus on environmental concerns and safety regulations. This led to the development of low-viscosity brake fluids that improved the responsiveness of anti-lock braking systems (ABS) and other advanced driver assistance technologies. Additionally, efforts were made to reduce the hygroscopic nature of brake fluids, as moisture absorption remained a persistent challenge affecting long-term performance and safety.
In recent years, the evolution of brake fluids has been driven by the need for higher boiling points to cope with the increased thermal loads generated by modern high-performance vehicles and regenerative braking systems in electric and hybrid vehicles. This has led to the development of advanced synthetic formulations, including those based on borate esters and other high-performance additives.
The current focus in brake fluid evolution is on the optimization of ester-based formulations, such as ethyl propanoate-ester brake fluids. These fluids offer a promising balance of high boiling points, low viscosity, and improved environmental compatibility. Research is ongoing to fine-tune the composition of these fluids to meet the demanding requirements of modern automotive systems while addressing concerns related to long-term stability, material compatibility, and environmental impact.
As vehicle technologies continue to advance, particularly with the rise of electric and autonomous vehicles, the evolution of brake fluids is expected to continue. Future developments may include smart fluids that can adapt their properties based on operating conditions, or even the integration of brake fluid monitoring systems to enhance safety and maintenance practices in next-generation vehicles.
The 1930s saw the introduction of glycol-based brake fluids, marking a significant advancement in brake system technology. These fluids offered improved thermal stability and higher boiling points, addressing many of the shortcomings of earlier formulations. The development of polyglycol-based fluids in the 1950s further enhanced brake fluid performance, particularly in terms of moisture resistance and compatibility with rubber components in the brake system.
As automotive technology progressed, so did the demands on brake fluid performance. The 1960s and 1970s witnessed the introduction of silicone-based brake fluids, which offered exceptional thermal stability and resistance to moisture absorption. However, their higher compressibility and incompatibility with conventional brake systems limited their widespread adoption.
The late 20th century brought about a focus on environmental concerns and safety regulations. This led to the development of low-viscosity brake fluids that improved the responsiveness of anti-lock braking systems (ABS) and other advanced driver assistance technologies. Additionally, efforts were made to reduce the hygroscopic nature of brake fluids, as moisture absorption remained a persistent challenge affecting long-term performance and safety.
In recent years, the evolution of brake fluids has been driven by the need for higher boiling points to cope with the increased thermal loads generated by modern high-performance vehicles and regenerative braking systems in electric and hybrid vehicles. This has led to the development of advanced synthetic formulations, including those based on borate esters and other high-performance additives.
The current focus in brake fluid evolution is on the optimization of ester-based formulations, such as ethyl propanoate-ester brake fluids. These fluids offer a promising balance of high boiling points, low viscosity, and improved environmental compatibility. Research is ongoing to fine-tune the composition of these fluids to meet the demanding requirements of modern automotive systems while addressing concerns related to long-term stability, material compatibility, and environmental impact.
As vehicle technologies continue to advance, particularly with the rise of electric and autonomous vehicles, the evolution of brake fluids is expected to continue. Future developments may include smart fluids that can adapt their properties based on operating conditions, or even the integration of brake fluid monitoring systems to enhance safety and maintenance practices in next-generation vehicles.
Market Demand Analysis
The market demand for optimized ethyl propanoate-ester brake fluid compositions has been steadily increasing in recent years, driven by the automotive industry's push for enhanced safety and performance. This trend is particularly evident in regions with stringent vehicle safety regulations, such as North America, Europe, and parts of Asia.
The global brake fluid market, which includes ethyl propanoate-ester based fluids, is experiencing robust growth. This expansion is primarily attributed to the rising production of vehicles worldwide, especially in emerging economies. The increasing adoption of advanced braking systems, such as anti-lock braking systems (ABS) and electronic stability control (ESC), further fuels the demand for high-performance brake fluids.
Ethyl propanoate-ester brake fluids are gaining traction due to their superior properties compared to traditional glycol-based fluids. These ester-based fluids offer improved thermal stability, higher boiling points, and better corrosion resistance. As vehicles become more sophisticated and operate under more demanding conditions, the need for brake fluids that can maintain their performance under extreme temperatures and pressures becomes critical.
The automotive aftermarket sector also contributes significantly to the demand for optimized brake fluid compositions. As vehicle owners become more aware of the importance of regular brake fluid maintenance, there is a growing market for high-quality replacement fluids that offer enhanced protection and longevity.
Environmental concerns are shaping market preferences as well. There is an increasing demand for brake fluids that are less toxic and more biodegradable. Ethyl propanoate-ester based fluids have the potential to meet these environmental requirements while maintaining high performance standards, making them attractive to both manufacturers and consumers.
The racing and high-performance vehicle segments represent niche markets with specific demands for brake fluid optimization. These sectors require fluids that can withstand extreme conditions and provide consistent performance under high stress, creating opportunities for specialized ethyl propanoate-ester formulations.
As electric and hybrid vehicles gain market share, there is a growing need for brake fluids compatible with regenerative braking systems. This presents both challenges and opportunities for ethyl propanoate-ester brake fluid compositions, as they must be adapted to meet the unique requirements of these new propulsion technologies.
The market analysis indicates that while demand is growing, there is also intense competition among brake fluid manufacturers. Companies are investing in research and development to create proprietary formulations that offer superior performance characteristics. This competitive landscape is driving innovation in the field of ethyl propanoate-ester brake fluids, with a focus on improving thermal stability, reducing moisture absorption, and enhancing overall braking efficiency.
The global brake fluid market, which includes ethyl propanoate-ester based fluids, is experiencing robust growth. This expansion is primarily attributed to the rising production of vehicles worldwide, especially in emerging economies. The increasing adoption of advanced braking systems, such as anti-lock braking systems (ABS) and electronic stability control (ESC), further fuels the demand for high-performance brake fluids.
Ethyl propanoate-ester brake fluids are gaining traction due to their superior properties compared to traditional glycol-based fluids. These ester-based fluids offer improved thermal stability, higher boiling points, and better corrosion resistance. As vehicles become more sophisticated and operate under more demanding conditions, the need for brake fluids that can maintain their performance under extreme temperatures and pressures becomes critical.
The automotive aftermarket sector also contributes significantly to the demand for optimized brake fluid compositions. As vehicle owners become more aware of the importance of regular brake fluid maintenance, there is a growing market for high-quality replacement fluids that offer enhanced protection and longevity.
Environmental concerns are shaping market preferences as well. There is an increasing demand for brake fluids that are less toxic and more biodegradable. Ethyl propanoate-ester based fluids have the potential to meet these environmental requirements while maintaining high performance standards, making them attractive to both manufacturers and consumers.
The racing and high-performance vehicle segments represent niche markets with specific demands for brake fluid optimization. These sectors require fluids that can withstand extreme conditions and provide consistent performance under high stress, creating opportunities for specialized ethyl propanoate-ester formulations.
As electric and hybrid vehicles gain market share, there is a growing need for brake fluids compatible with regenerative braking systems. This presents both challenges and opportunities for ethyl propanoate-ester brake fluid compositions, as they must be adapted to meet the unique requirements of these new propulsion technologies.
The market analysis indicates that while demand is growing, there is also intense competition among brake fluid manufacturers. Companies are investing in research and development to create proprietary formulations that offer superior performance characteristics. This competitive landscape is driving innovation in the field of ethyl propanoate-ester brake fluids, with a focus on improving thermal stability, reducing moisture absorption, and enhancing overall braking efficiency.
Current Challenges
The optimization of ethyl propanoate-ester brake fluid composition faces several significant challenges in the current technological landscape. One of the primary obstacles is achieving the right balance between performance characteristics and environmental sustainability. As regulatory pressures increase globally, there is a growing demand for brake fluids that maintain high performance while minimizing environmental impact.
A major technical hurdle lies in improving the thermal stability of ethyl propanoate-ester based brake fluids. While these fluids offer advantages in terms of their high boiling point and low hygroscopicity, they can still undergo thermal degradation under extreme operating conditions. This degradation can lead to a decrease in brake system efficiency and potentially compromise safety.
Compatibility with existing brake system components presents another challenge. As the automotive industry transitions towards more sustainable solutions, it is crucial that new brake fluid formulations remain compatible with current rubber seals, hoses, and other materials used in brake systems. Any incompatibility could lead to premature wear, leakage, or failure of critical components.
The viscosity-temperature relationship of ethyl propanoate-ester brake fluids also requires further optimization. Maintaining appropriate viscosity across a wide temperature range is essential for consistent brake performance in various climatic conditions. Current formulations may struggle to provide optimal viscosity characteristics at extreme temperatures, particularly in very cold environments.
Corrosion resistance is another area that demands attention. While ester-based fluids generally offer good corrosion protection, there is room for improvement, especially when considering long-term exposure to moisture and other contaminants. Enhancing the corrosion inhibition properties of the fluid without compromising other performance attributes remains a significant challenge.
Cost-effectiveness is a persistent issue in the development of advanced brake fluid compositions. The production of high-quality ethyl propanoate-ester brake fluids often involves complex synthesis processes and expensive raw materials. Finding ways to reduce production costs while maintaining or improving performance is crucial for widespread adoption in the automotive industry.
Lastly, the long-term stability and shelf life of these brake fluids pose ongoing challenges. Ensuring that the fluid maintains its properties over extended periods, both in storage and in use, is critical for safety and reliability. This includes resistance to oxidation, hydrolysis, and other forms of chemical degradation that could affect the fluid's performance over time.
A major technical hurdle lies in improving the thermal stability of ethyl propanoate-ester based brake fluids. While these fluids offer advantages in terms of their high boiling point and low hygroscopicity, they can still undergo thermal degradation under extreme operating conditions. This degradation can lead to a decrease in brake system efficiency and potentially compromise safety.
Compatibility with existing brake system components presents another challenge. As the automotive industry transitions towards more sustainable solutions, it is crucial that new brake fluid formulations remain compatible with current rubber seals, hoses, and other materials used in brake systems. Any incompatibility could lead to premature wear, leakage, or failure of critical components.
The viscosity-temperature relationship of ethyl propanoate-ester brake fluids also requires further optimization. Maintaining appropriate viscosity across a wide temperature range is essential for consistent brake performance in various climatic conditions. Current formulations may struggle to provide optimal viscosity characteristics at extreme temperatures, particularly in very cold environments.
Corrosion resistance is another area that demands attention. While ester-based fluids generally offer good corrosion protection, there is room for improvement, especially when considering long-term exposure to moisture and other contaminants. Enhancing the corrosion inhibition properties of the fluid without compromising other performance attributes remains a significant challenge.
Cost-effectiveness is a persistent issue in the development of advanced brake fluid compositions. The production of high-quality ethyl propanoate-ester brake fluids often involves complex synthesis processes and expensive raw materials. Finding ways to reduce production costs while maintaining or improving performance is crucial for widespread adoption in the automotive industry.
Lastly, the long-term stability and shelf life of these brake fluids pose ongoing challenges. Ensuring that the fluid maintains its properties over extended periods, both in storage and in use, is critical for safety and reliability. This includes resistance to oxidation, hydrolysis, and other forms of chemical degradation that could affect the fluid's performance over time.
Existing Formulations
01 Composition of ester-based brake fluids
Ester-based brake fluids, including those containing ethyl propanoate, are formulated to provide improved performance characteristics. These compositions often include a mixture of different esters, along with additives to enhance stability, lubricity, and corrosion resistance. The specific combination of esters and additives is tailored to meet the requirements of modern brake systems.- Composition of ester-based brake fluids: Ester-based brake fluids, including those containing ethyl propanoate, are formulated to provide improved performance characteristics. These compositions often include a mixture of different esters, along with additives to enhance stability, lubricity, and corrosion resistance. The specific blend of esters and additives is tailored to meet the requirements of modern braking systems.
- Additives for enhancing brake fluid properties: Various additives are incorporated into ester-based brake fluids to improve their performance. These may include antioxidants, corrosion inhibitors, anti-wear agents, and viscosity modifiers. The selection and concentration of additives are crucial for maintaining the fluid's effectiveness under diverse operating conditions and extending the lifespan of brake system components.
- Environmental and safety considerations: Development of ester-based brake fluids, including those with ethyl propanoate, focuses on improving environmental compatibility and safety. This includes formulations with reduced toxicity, enhanced biodegradability, and lower vapor pressure to minimize environmental impact and improve handling safety. These fluids are designed to meet or exceed regulatory standards for automotive brake fluids.
- Performance under extreme conditions: Ester-based brake fluids are engineered to maintain performance under extreme temperature and pressure conditions. This includes optimizing the fluid's boiling point, viscosity index, and thermal stability to ensure reliable braking performance in both high-temperature and low-temperature environments. The composition is tailored to prevent vapor lock and maintain hydraulic efficiency across a wide range of operating conditions.
- Manufacturing and quality control processes: The production of ester-based brake fluids involves precise manufacturing processes and stringent quality control measures. This includes careful selection and purification of raw materials, controlled esterification reactions, and blending procedures. Advanced testing methods are employed to ensure consistency, purity, and compliance with industry standards, guaranteeing the reliability and performance of the final product.
02 Additives for enhancing brake fluid properties
Various additives are incorporated into ester-based brake fluid compositions to improve their performance. These may include antioxidants, corrosion inhibitors, anti-wear agents, and viscosity modifiers. The selection and concentration of additives are crucial in achieving the desired properties such as thermal stability, low-temperature fluidity, and compatibility with brake system components.Expand Specific Solutions03 Manufacturing processes for ester-based brake fluids
The production of ester-based brake fluids, including those with ethyl propanoate, involves specific manufacturing processes. These may include controlled esterification reactions, blending of different esters, and incorporation of additives under precise conditions. The manufacturing process is designed to ensure consistent quality and performance of the final brake fluid product.Expand Specific Solutions04 Environmental and safety considerations
Development of ester-based brake fluids takes into account environmental and safety factors. This includes considerations for biodegradability, toxicity, and disposal of used brake fluids. Formulations may be designed to minimize environmental impact while maintaining high performance standards required for automotive safety.Expand Specific Solutions05 Performance testing and quality control
Rigorous testing protocols are employed to evaluate the performance and quality of ester-based brake fluids. This includes tests for boiling point, viscosity at various temperatures, corrosion protection, and compatibility with brake system materials. Quality control measures are implemented throughout the production process to ensure consistency and reliability of the brake fluid composition.Expand Specific Solutions
Key Industry Players
The optimization of ethyl propanoate-ester brake fluid composition is in a mature stage of development, with a significant market presence and established technological foundations. The global brake fluid market size is substantial, estimated to be worth several billion dollars annually. Key players in this field include major automotive suppliers and chemical companies such as Hyundai Mobis, Dow Global Technologies, NSK Ltd., and BASF Corp. These companies have demonstrated advanced technical capabilities in developing and refining brake fluid formulations. The technology's maturity is evident from the involvement of diverse industry leaders, including automotive manufacturers like Hyundai Motor Co. and Kia Corp., as well as specialized chemical firms like Clariant International AG and Kuraray Co., Ltd., indicating a well-established ecosystem of suppliers and end-users.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies LLC has developed a high-performance ethyl propanoate-ester brake fluid composition focusing on molecular engineering and advanced polymer science. Their approach utilizes custom-designed ethyl propanoate esters with optimized molecular structures to enhance thermal stability and reduce vapor lock tendencies. Dow has incorporated proprietary polymeric additives that act as viscosity index improvers, ensuring consistent fluid performance across a wide temperature range[1]. The company's formulation also features advanced corrosion inhibitors based on organometallic compounds, providing superior protection for both ferrous and non-ferrous brake system components[3]. To address environmental concerns, Dow has implemented a green chemistry approach, utilizing bio-based feedstocks for a portion of the ester production and developing additives with improved biodegradability profiles[5]. The company employs advanced computational fluid dynamics simulations to predict and optimize the brake fluid's behavior under various operating conditions, leading to a more robust and reliable formulation[7].
Strengths: Molecularly engineered for optimal performance, excellent corrosion protection, and improved environmental profile. Weaknesses: May require specialized manufacturing processes and potentially higher production costs due to advanced formulation techniques.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to optimizing ethyl propanoate-ester brake fluid composition. Their solution involves a multi-component system that combines ethyl propanoate esters with carefully selected additives to enhance performance and stability. The company utilizes a proprietary blend of antioxidants and corrosion inhibitors to extend the fluid's service life and protect brake system components. BASF's formulation also incorporates advanced polymeric viscosity modifiers to maintain optimal viscosity across a wide temperature range, ensuring consistent brake performance in various operating conditions[1][3]. The company has implemented a rigorous testing protocol, including accelerated aging tests and compatibility studies with different elastomers used in brake systems, to validate the long-term effectiveness of their brake fluid composition[5].
Strengths: Superior thermal stability, extended service life, and broad temperature performance range. Weaknesses: Potentially higher production costs due to complex formulation and extensive testing requirements.
Key Innovations
Hydraulic fluid with improved properties
PatentInactiveEP0028789A1
Innovation
- A hydraulic fluid composition comprising 20-40 wt% ethylene glycol monoalkyl ether, 30-60% bis(ethylene glycol monoalkyl ether) formal, 0.1-5% alkylamine, and 0.05-5% stabilizers/inhibitors, specifically formulated to enhance thermal stability, viscosity consistency, and polymer compatibility, with a focus on achieving superior performance in evaporation tests.
Novel functional fluid composition
PatentWO2015052234A1
Innovation
- A functional fluid composition comprising alkoxy glycol borate esters, alkoxy glycol components, and an alkoxylate of a saturated or unsaturated hydroxy-substituted fatty acid or its ester, combined with corrosion inhibition additives, which provides superior lubricity, low temperature kinematic viscosity, and corrosion resistance.
Environmental Impact
The environmental impact of ethyl propanoate-ester brake fluid composition is a critical consideration in the optimization process. As automotive manufacturers and regulatory bodies increasingly prioritize sustainability, the environmental footprint of brake fluid formulations has come under scrutiny. Ethyl propanoate-ester based brake fluids offer several advantages in this regard, particularly when compared to traditional glycol-based fluids.
One of the primary environmental benefits of ethyl propanoate-ester brake fluids is their biodegradability. These fluids break down more readily in the environment, reducing the long-term impact of leaks or spills. This characteristic is particularly important in minimizing soil and water contamination, which can occur during vehicle maintenance or accidents. The faster degradation rate also means less accumulation of harmful substances in ecosystems over time.
Volatility is another factor that influences the environmental impact of brake fluids. Ethyl propanoate-ester formulations generally have lower volatility compared to some conventional alternatives. This reduced volatility translates to lower emissions of volatile organic compounds (VOCs) into the atmosphere. VOCs contribute to air pollution and can have adverse effects on both human health and the environment, making the lower emission profile of ethyl propanoate-ester fluids an important environmental advantage.
The production process of ethyl propanoate-ester brake fluids also plays a role in their overall environmental impact. These fluids can be synthesized using renewable resources, potentially reducing the carbon footprint associated with their manufacture. The use of bio-based feedstocks aligns with the growing trend towards sustainable and circular economy principles in the automotive industry.
However, it is important to note that the environmental benefits of ethyl propanoate-ester brake fluids must be balanced against their performance characteristics. Any optimization of the fluid composition must ensure that the resulting product meets or exceeds safety and performance standards. This includes factors such as boiling point, viscosity, and corrosion protection, which are critical for brake system functionality and longevity.
The disposal of brake fluid at the end of its lifecycle is another environmental consideration. While ethyl propanoate-ester fluids may offer advantages in terms of biodegradability, proper disposal practices are still essential to minimize environmental impact. Recycling and responsible waste management strategies should be developed and implemented alongside the use of these more environmentally friendly formulations.
In conclusion, the optimization of ethyl propanoate-ester brake fluid composition presents an opportunity to significantly reduce the environmental impact of automotive brake systems. By focusing on biodegradability, reduced volatility, sustainable production methods, and effective end-of-life management, manufacturers can develop brake fluid formulations that not only meet performance requirements but also contribute to more sustainable transportation solutions.
One of the primary environmental benefits of ethyl propanoate-ester brake fluids is their biodegradability. These fluids break down more readily in the environment, reducing the long-term impact of leaks or spills. This characteristic is particularly important in minimizing soil and water contamination, which can occur during vehicle maintenance or accidents. The faster degradation rate also means less accumulation of harmful substances in ecosystems over time.
Volatility is another factor that influences the environmental impact of brake fluids. Ethyl propanoate-ester formulations generally have lower volatility compared to some conventional alternatives. This reduced volatility translates to lower emissions of volatile organic compounds (VOCs) into the atmosphere. VOCs contribute to air pollution and can have adverse effects on both human health and the environment, making the lower emission profile of ethyl propanoate-ester fluids an important environmental advantage.
The production process of ethyl propanoate-ester brake fluids also plays a role in their overall environmental impact. These fluids can be synthesized using renewable resources, potentially reducing the carbon footprint associated with their manufacture. The use of bio-based feedstocks aligns with the growing trend towards sustainable and circular economy principles in the automotive industry.
However, it is important to note that the environmental benefits of ethyl propanoate-ester brake fluids must be balanced against their performance characteristics. Any optimization of the fluid composition must ensure that the resulting product meets or exceeds safety and performance standards. This includes factors such as boiling point, viscosity, and corrosion protection, which are critical for brake system functionality and longevity.
The disposal of brake fluid at the end of its lifecycle is another environmental consideration. While ethyl propanoate-ester fluids may offer advantages in terms of biodegradability, proper disposal practices are still essential to minimize environmental impact. Recycling and responsible waste management strategies should be developed and implemented alongside the use of these more environmentally friendly formulations.
In conclusion, the optimization of ethyl propanoate-ester brake fluid composition presents an opportunity to significantly reduce the environmental impact of automotive brake systems. By focusing on biodegradability, reduced volatility, sustainable production methods, and effective end-of-life management, manufacturers can develop brake fluid formulations that not only meet performance requirements but also contribute to more sustainable transportation solutions.
Safety Regulations
Safety regulations play a crucial role in the development and implementation of brake fluid compositions, particularly for ethyl propanoate-ester based formulations. These regulations are designed to ensure the safety and reliability of brake systems in various vehicles, protecting both drivers and passengers.
The primary regulatory bodies overseeing brake fluid safety standards include the United States Department of Transportation (DOT), the European Union's Economic Commission for Europe (ECE), and the International Organization for Standardization (ISO). These organizations have established comprehensive guidelines and testing procedures to evaluate the performance and safety characteristics of brake fluids.
For ethyl propanoate-ester brake fluids, safety regulations focus on several key parameters. Boiling point is a critical factor, as it determines the fluid's ability to resist vaporization under high temperatures generated during braking. Regulations typically specify minimum dry and wet boiling points to ensure consistent performance under various conditions. Viscosity requirements are also crucial, as the fluid must maintain proper flow characteristics across a wide temperature range to ensure reliable brake operation in both cold and hot environments.
Corrosion protection is another vital aspect addressed by safety regulations. Brake fluids must demonstrate compatibility with various metals and elastomers used in brake systems to prevent degradation of components over time. Standardized tests evaluate the fluid's ability to inhibit corrosion on metals such as steel, aluminum, cast iron, and copper alloys.
Water tolerance is a significant concern for brake fluid safety, as moisture absorption can lead to decreased boiling points and potential brake failure. Regulations mandate specific water content limits and require testing of hygroscopic properties to ensure the fluid maintains its performance characteristics even when exposed to moisture over time.
Stability and aging characteristics are also regulated to guarantee long-term reliability. Accelerated aging tests simulate extended use under various conditions to assess the fluid's ability to maintain its properties over the vehicle's lifespan. These tests evaluate factors such as oxidation resistance, thermal stability, and chemical compatibility with brake system components.
Toxicity and environmental impact considerations have become increasingly important in recent years. Safety regulations now often include requirements for biodegradability and restrictions on the use of certain harmful substances in brake fluid formulations. This shift reflects growing concerns about the environmental impact of automotive fluids and the need for more sustainable solutions.
Compliance with these safety regulations is mandatory for manufacturers and distributors of brake fluids. Rigorous testing and certification processes are required to demonstrate that a product meets all applicable standards before it can be marketed and sold. Regular audits and quality control measures are typically implemented to ensure ongoing compliance and maintain product safety.
The primary regulatory bodies overseeing brake fluid safety standards include the United States Department of Transportation (DOT), the European Union's Economic Commission for Europe (ECE), and the International Organization for Standardization (ISO). These organizations have established comprehensive guidelines and testing procedures to evaluate the performance and safety characteristics of brake fluids.
For ethyl propanoate-ester brake fluids, safety regulations focus on several key parameters. Boiling point is a critical factor, as it determines the fluid's ability to resist vaporization under high temperatures generated during braking. Regulations typically specify minimum dry and wet boiling points to ensure consistent performance under various conditions. Viscosity requirements are also crucial, as the fluid must maintain proper flow characteristics across a wide temperature range to ensure reliable brake operation in both cold and hot environments.
Corrosion protection is another vital aspect addressed by safety regulations. Brake fluids must demonstrate compatibility with various metals and elastomers used in brake systems to prevent degradation of components over time. Standardized tests evaluate the fluid's ability to inhibit corrosion on metals such as steel, aluminum, cast iron, and copper alloys.
Water tolerance is a significant concern for brake fluid safety, as moisture absorption can lead to decreased boiling points and potential brake failure. Regulations mandate specific water content limits and require testing of hygroscopic properties to ensure the fluid maintains its performance characteristics even when exposed to moisture over time.
Stability and aging characteristics are also regulated to guarantee long-term reliability. Accelerated aging tests simulate extended use under various conditions to assess the fluid's ability to maintain its properties over the vehicle's lifespan. These tests evaluate factors such as oxidation resistance, thermal stability, and chemical compatibility with brake system components.
Toxicity and environmental impact considerations have become increasingly important in recent years. Safety regulations now often include requirements for biodegradability and restrictions on the use of certain harmful substances in brake fluid formulations. This shift reflects growing concerns about the environmental impact of automotive fluids and the need for more sustainable solutions.
Compliance with these safety regulations is mandatory for manufacturers and distributors of brake fluids. Rigorous testing and certification processes are required to demonstrate that a product meets all applicable standards before it can be marketed and sold. Regular audits and quality control measures are typically implemented to ensure ongoing compliance and maintain product safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!