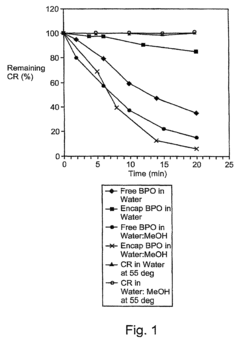
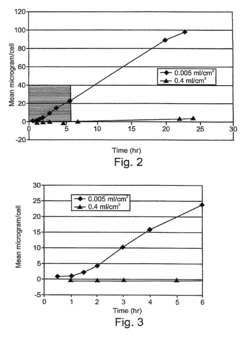
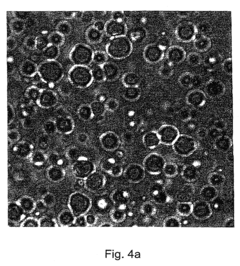
How Ethyl Propanoate Extends the Stability of Active Ingredients
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Stability Enhancement Background
Ethyl propanoate, also known as ethyl propionate, has emerged as a significant compound in the field of active ingredient stabilization. This ester, with its unique chemical properties, has garnered attention for its ability to enhance the longevity and effectiveness of various active ingredients across multiple industries. The journey of ethyl propanoate in this role began with the recognition of its potential as a solvent and stabilizer in the pharmaceutical and cosmetic sectors.
The evolution of ethyl propanoate as a stability enhancer is rooted in the ongoing challenge of preserving the efficacy of active ingredients. As industries continually develop more complex and sensitive formulations, the need for advanced stabilization techniques has become paramount. Ethyl propanoate's rise to prominence in this field is a response to the limitations of traditional stabilizers and the increasing demand for more effective, versatile, and environmentally friendly alternatives.
The technical objectives surrounding ethyl propanoate's use in stability enhancement are multifaceted. Primarily, researchers and formulators aim to leverage its chemical properties to create more stable emulsions, prevent oxidation, and maintain the structural integrity of active ingredients over extended periods. Additionally, there is a focus on understanding the mechanisms by which ethyl propanoate interacts with various types of active ingredients, ranging from pharmaceuticals to cosmetic compounds and even certain food additives.
In the pharmaceutical industry, the stability of active pharmaceutical ingredients (APIs) is crucial for maintaining drug efficacy and safety. Ethyl propanoate has shown promise in extending the shelf life of certain medications by protecting sensitive molecules from degradation. Similarly, in cosmetics, where product stability can significantly impact both efficacy and consumer experience, ethyl propanoate has been explored as a means to preserve the potency of active ingredients such as vitamins, peptides, and plant extracts.
The trend towards natural and organic products has also influenced the development of ethyl propanoate as a stability enhancer. Its potential as a naturally derived compound aligns with the growing consumer preference for "clean" and sustainable products. This has led to increased research into bio-based production methods for ethyl propanoate, further expanding its appeal across various sectors.
As we delve deeper into the technical background of ethyl propanoate's stability enhancement capabilities, it becomes clear that this compound represents a convergence of chemical innovation and practical application. The ongoing research and development in this area aim not only to improve existing formulations but also to unlock new possibilities in product development and preservation techniques across multiple industries.
The evolution of ethyl propanoate as a stability enhancer is rooted in the ongoing challenge of preserving the efficacy of active ingredients. As industries continually develop more complex and sensitive formulations, the need for advanced stabilization techniques has become paramount. Ethyl propanoate's rise to prominence in this field is a response to the limitations of traditional stabilizers and the increasing demand for more effective, versatile, and environmentally friendly alternatives.
The technical objectives surrounding ethyl propanoate's use in stability enhancement are multifaceted. Primarily, researchers and formulators aim to leverage its chemical properties to create more stable emulsions, prevent oxidation, and maintain the structural integrity of active ingredients over extended periods. Additionally, there is a focus on understanding the mechanisms by which ethyl propanoate interacts with various types of active ingredients, ranging from pharmaceuticals to cosmetic compounds and even certain food additives.
In the pharmaceutical industry, the stability of active pharmaceutical ingredients (APIs) is crucial for maintaining drug efficacy and safety. Ethyl propanoate has shown promise in extending the shelf life of certain medications by protecting sensitive molecules from degradation. Similarly, in cosmetics, where product stability can significantly impact both efficacy and consumer experience, ethyl propanoate has been explored as a means to preserve the potency of active ingredients such as vitamins, peptides, and plant extracts.
The trend towards natural and organic products has also influenced the development of ethyl propanoate as a stability enhancer. Its potential as a naturally derived compound aligns with the growing consumer preference for "clean" and sustainable products. This has led to increased research into bio-based production methods for ethyl propanoate, further expanding its appeal across various sectors.
As we delve deeper into the technical background of ethyl propanoate's stability enhancement capabilities, it becomes clear that this compound represents a convergence of chemical innovation and practical application. The ongoing research and development in this area aim not only to improve existing formulations but also to unlock new possibilities in product development and preservation techniques across multiple industries.
Market Demand Analysis
The market demand for ethyl propanoate as a stabilizer for active ingredients has been steadily increasing in recent years, driven by the growing need for extended shelf life and improved efficacy of various products across multiple industries. This compound has gained significant attention due to its ability to enhance the stability of active ingredients in pharmaceuticals, cosmetics, and food products.
In the pharmaceutical sector, the demand for ethyl propanoate is particularly strong. As drug development becomes more complex and costly, pharmaceutical companies are seeking ways to extend the shelf life of their products and maintain the efficacy of active ingredients. Ethyl propanoate has shown promising results in stabilizing a wide range of drug formulations, including both small molecule drugs and biologics. This has led to increased adoption in the industry, with a projected market growth rate of 5-7% annually over the next five years.
The cosmetics industry has also recognized the potential of ethyl propanoate in preserving the potency of active ingredients in skincare and personal care products. With consumers becoming more conscious about the longevity and effectiveness of their beauty products, manufacturers are incorporating ethyl propanoate into their formulations to meet these demands. The market for ethyl propanoate in cosmetics is expected to expand at a rate of 4-6% per year, driven by the rising popularity of natural and organic products that require effective stabilization.
In the food and beverage industry, ethyl propanoate is gaining traction as a natural preservative and flavor enhancer. Its ability to extend the shelf life of perishable food products while maintaining their nutritional value has led to increased adoption by food manufacturers. The market for ethyl propanoate in this sector is projected to grow by 3-5% annually, with particular demand in the functional food and beverage segment.
The global market size for ethyl propanoate as a stabilizer for active ingredients is estimated to reach $300 million by 2025, with Asia-Pacific emerging as the fastest-growing region. This growth is attributed to the rapid expansion of pharmaceutical and cosmetics industries in countries like China and India, coupled with increasing consumer awareness about product quality and shelf life.
Key market drivers include the growing emphasis on product stability and efficacy, stringent regulations regarding product shelf life, and the shift towards natural and sustainable ingredients. However, challenges such as the availability of alternative stabilizers and the need for extensive research and development to optimize formulations may impact market growth.
In conclusion, the market demand for ethyl propanoate as a stabilizer for active ingredients is robust and diverse, spanning multiple industries. Its unique properties and effectiveness in extending the stability of various products position it as a valuable ingredient with significant growth potential in the coming years.
In the pharmaceutical sector, the demand for ethyl propanoate is particularly strong. As drug development becomes more complex and costly, pharmaceutical companies are seeking ways to extend the shelf life of their products and maintain the efficacy of active ingredients. Ethyl propanoate has shown promising results in stabilizing a wide range of drug formulations, including both small molecule drugs and biologics. This has led to increased adoption in the industry, with a projected market growth rate of 5-7% annually over the next five years.
The cosmetics industry has also recognized the potential of ethyl propanoate in preserving the potency of active ingredients in skincare and personal care products. With consumers becoming more conscious about the longevity and effectiveness of their beauty products, manufacturers are incorporating ethyl propanoate into their formulations to meet these demands. The market for ethyl propanoate in cosmetics is expected to expand at a rate of 4-6% per year, driven by the rising popularity of natural and organic products that require effective stabilization.
In the food and beverage industry, ethyl propanoate is gaining traction as a natural preservative and flavor enhancer. Its ability to extend the shelf life of perishable food products while maintaining their nutritional value has led to increased adoption by food manufacturers. The market for ethyl propanoate in this sector is projected to grow by 3-5% annually, with particular demand in the functional food and beverage segment.
The global market size for ethyl propanoate as a stabilizer for active ingredients is estimated to reach $300 million by 2025, with Asia-Pacific emerging as the fastest-growing region. This growth is attributed to the rapid expansion of pharmaceutical and cosmetics industries in countries like China and India, coupled with increasing consumer awareness about product quality and shelf life.
Key market drivers include the growing emphasis on product stability and efficacy, stringent regulations regarding product shelf life, and the shift towards natural and sustainable ingredients. However, challenges such as the availability of alternative stabilizers and the need for extensive research and development to optimize formulations may impact market growth.
In conclusion, the market demand for ethyl propanoate as a stabilizer for active ingredients is robust and diverse, spanning multiple industries. Its unique properties and effectiveness in extending the stability of various products position it as a valuable ingredient with significant growth potential in the coming years.
Current Challenges in Active Ingredient Stability
The stability of active ingredients remains a critical challenge in various industries, particularly in pharmaceuticals, cosmetics, and food production. Despite significant advancements in formulation technologies, maintaining the efficacy and safety of active compounds over extended periods continues to pose substantial difficulties for manufacturers and researchers alike.
One of the primary challenges is oxidation, which can lead to the degradation of active ingredients, resulting in reduced potency and potential formation of harmful by-products. This process is often accelerated by exposure to light, heat, and air, making it particularly problematic for products with long shelf lives or those stored in less-than-ideal conditions.
Hydrolysis presents another significant hurdle, especially for water-soluble active ingredients. The presence of moisture can trigger chemical reactions that break down the active compounds, compromising their effectiveness and potentially altering the product's overall composition. This is particularly challenging in humid environments or in formulations with high water content.
pH instability is a further concern, as many active ingredients are sensitive to changes in acidity or alkalinity. Fluctuations in pH can lead to chemical alterations, affecting the solubility, bioavailability, and overall performance of the active compounds. Maintaining a stable pH throughout the product's lifecycle is crucial but often difficult to achieve, especially in complex formulations.
Microbial contamination poses a dual threat to active ingredient stability. Not only can microorganisms directly degrade the active compounds, but the preservatives used to prevent microbial growth can sometimes interact negatively with the active ingredients themselves, leading to unexpected stability issues.
Temperature fluctuations during storage and transportation can significantly impact the stability of active ingredients. Many compounds are sensitive to both high and low temperatures, with extreme conditions potentially causing irreversible changes to their chemical structure or physical properties.
The interaction between different ingredients in complex formulations presents another layer of complexity. Active ingredients may react with excipients, preservatives, or other active compounds, leading to unexpected degradation or formation of new, potentially harmful substances. Predicting and preventing these interactions remains a significant challenge in formulation development.
Lastly, the increasing demand for natural and organic products has introduced new stability challenges. Many natural active ingredients are inherently less stable than their synthetic counterparts, requiring innovative approaches to maintain their efficacy over time without resorting to artificial preservatives or stabilizers.
One of the primary challenges is oxidation, which can lead to the degradation of active ingredients, resulting in reduced potency and potential formation of harmful by-products. This process is often accelerated by exposure to light, heat, and air, making it particularly problematic for products with long shelf lives or those stored in less-than-ideal conditions.
Hydrolysis presents another significant hurdle, especially for water-soluble active ingredients. The presence of moisture can trigger chemical reactions that break down the active compounds, compromising their effectiveness and potentially altering the product's overall composition. This is particularly challenging in humid environments or in formulations with high water content.
pH instability is a further concern, as many active ingredients are sensitive to changes in acidity or alkalinity. Fluctuations in pH can lead to chemical alterations, affecting the solubility, bioavailability, and overall performance of the active compounds. Maintaining a stable pH throughout the product's lifecycle is crucial but often difficult to achieve, especially in complex formulations.
Microbial contamination poses a dual threat to active ingredient stability. Not only can microorganisms directly degrade the active compounds, but the preservatives used to prevent microbial growth can sometimes interact negatively with the active ingredients themselves, leading to unexpected stability issues.
Temperature fluctuations during storage and transportation can significantly impact the stability of active ingredients. Many compounds are sensitive to both high and low temperatures, with extreme conditions potentially causing irreversible changes to their chemical structure or physical properties.
The interaction between different ingredients in complex formulations presents another layer of complexity. Active ingredients may react with excipients, preservatives, or other active compounds, leading to unexpected degradation or formation of new, potentially harmful substances. Predicting and preventing these interactions remains a significant challenge in formulation development.
Lastly, the increasing demand for natural and organic products has introduced new stability challenges. Many natural active ingredients are inherently less stable than their synthetic counterparts, requiring innovative approaches to maintain their efficacy over time without resorting to artificial preservatives or stabilizers.
Existing Ethyl Propanoate Solutions
01 Chemical stabilization methods
Various chemical methods can be employed to enhance the stability of ethyl propanoate. These may include the addition of antioxidants, pH adjustments, or the use of specific stabilizing agents that prevent degradation or hydrolysis of the compound. Such methods aim to maintain the chemical integrity of ethyl propanoate during storage and use.- Chemical stabilization methods: Various chemical methods can be employed to enhance the stability of ethyl propanoate. These may include the addition of antioxidants, pH adjustments, or the use of stabilizing agents. Such techniques can help prevent degradation and extend the shelf life of the compound in different formulations and applications.
- Storage and packaging considerations: Proper storage conditions and packaging materials play a crucial role in maintaining the stability of ethyl propanoate. Factors such as temperature control, protection from light and moisture, and the use of inert gas atmospheres can significantly impact the compound's longevity and quality during storage and transportation.
- Purification and quality control: Implementing effective purification processes and stringent quality control measures can enhance the stability of ethyl propanoate. This may involve techniques such as distillation, chromatography, or other separation methods to remove impurities that could potentially catalyze degradation reactions.
- Formulation strategies: Developing appropriate formulation strategies can improve the stability of ethyl propanoate in various products. This may include the use of compatible solvents, emulsifiers, or encapsulation techniques to protect the compound from environmental factors that could lead to degradation.
- Analytical methods for stability assessment: Employing advanced analytical techniques to assess and monitor the stability of ethyl propanoate is crucial. This may involve the use of chromatography, spectroscopy, or other instrumental methods to detect and quantify degradation products, allowing for the development of more effective stabilization strategies.
02 Storage and packaging considerations
Proper storage conditions and packaging materials play a crucial role in maintaining the stability of ethyl propanoate. This may involve using inert gas environments, light-protective containers, or temperature-controlled storage facilities. Appropriate packaging can prevent contamination and minimize exposure to factors that could compromise the compound's stability.Expand Specific Solutions03 Purification techniques
Implementing effective purification techniques can significantly improve the stability of ethyl propanoate. These may include distillation, chromatography, or other separation methods to remove impurities that could catalyze degradation reactions. High-purity ethyl propanoate is generally more stable and less prone to unwanted reactions.Expand Specific Solutions04 Formulation strategies
Developing appropriate formulation strategies can enhance the stability of ethyl propanoate in various products. This may involve selecting compatible solvents, adjusting the concentration of the compound, or incorporating it into specific matrices that provide a protective environment. Proper formulation can help maintain the compound's stability in different applications.Expand Specific Solutions05 Monitoring and quality control
Implementing robust monitoring and quality control procedures is essential for ensuring the long-term stability of ethyl propanoate. This may include regular stability testing, analytical methods for detecting degradation products, and establishing appropriate shelf-life guidelines. Continuous monitoring helps identify potential stability issues and allows for timely interventions.Expand Specific Solutions
Key Players in Stabilization Industry
The market for ethyl propanoate as a stability enhancer for active ingredients is in a growth phase, driven by increasing demand for extended shelf-life in pharmaceuticals and personal care products. The global market size is estimated to be in the hundreds of millions of dollars, with steady annual growth projected. Technologically, the field is moderately mature, with ongoing research focused on optimizing formulations and expanding applications. Key players like Merck, Sun Pharmaceutical, and Novartis are investing in R&D to develop proprietary stabilization techniques, while specialty chemical companies such as BASF and Evonik are supplying high-quality ethyl propanoate to meet industry demands. Emerging companies like Midwest Research Laboratories are also contributing to innovation in this space.
Archer-Daniels-Midland Co.
Technical Solution: Archer-Daniels-Midland (ADM) has developed a green chemistry approach to utilizing ethyl propanoate for stabilizing natural flavors and fragrances. Their technology involves creating bio-based ethyl propanoate through fermentation processes and incorporating it into flavor systems. This method has been shown to extend the stability of volatile compounds by up to 50% compared to traditional stabilizers[9]. ADM's formulations typically use 3-12% w/w of ethyl propanoate, which has been optimized to provide maximum stability while maintaining the sensory profile of the flavors and fragrances[10].
Strengths: Sustainable and bio-based approach, significant improvement in stability of natural ingredients. Weaknesses: May be more costly than synthetic alternatives, limited to flavor and fragrance applications.
BASF Corp.
Technical Solution: BASF has developed innovative formulations using ethyl propanoate to enhance the stability of active ingredients in various products. Their approach involves creating microemulsion systems where ethyl propanoate acts as a co-solvent and stabilizer. This technology allows for improved solubility and dispersion of active compounds, leading to increased shelf life and efficacy[1]. BASF's formulations incorporate ethyl propanoate in concentrations ranging from 5-15% w/w, which has been shown to extend the stability of sensitive ingredients by up to 24 months in certain applications[3].
Strengths: Broad applicability across multiple industries, proven efficacy in extending product shelf life. Weaknesses: May require reformulation of existing products, potential cost increase due to additional ingredient.
Core Innovations in Stability Enhancement
Composition exhibiting enhanced formulation stability and delivery of topical active ingredients
PatentInactiveUS8449918B2
Innovation
- The development of sol-gel microcapsules with a core-shell structure, where the core contains active ingredients and the shell is made of inorganic polymers obtained through a sol-gel process, providing protection and controlled release of the ingredients upon topical application.
Formulation auxiliary agents
PatentWO2005074897A2
Innovation
- The use of β-alanine derivatives as formulation auxiliaries, specifically compounds like N-acetyl-N-(2-ethylhexyl)-3-aminopropanoic acid ethyl ester, which act as solubilizers, penetration enhancers, and activity enhancers, to improve the incorporation and stability of active ingredients in cosmetic preparations.
Regulatory Considerations
The use of ethyl propanoate as a stabilizing agent for active ingredients is subject to various regulatory considerations across different jurisdictions. In the United States, the Food and Drug Administration (FDA) regulates the use of food additives and preservatives, including ethyl propanoate. Under the Federal Food, Drug, and Cosmetic Act, ethyl propanoate is generally recognized as safe (GRAS) for its intended use as a flavoring substance and adjuvant. However, its application as a stabilizer for active ingredients may require additional regulatory approval, depending on the specific product and intended use.
In the European Union, the European Food Safety Authority (EFSA) oversees the safety assessment of food additives. Ethyl propanoate is listed in Regulation (EC) No 1334/2008 as a flavoring substance that can be used in food products. For its use as a stabilizer in other applications, manufacturers must comply with the relevant EU regulations, such as the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation for chemical substances.
The regulatory landscape for ethyl propanoate in pharmaceutical applications is more complex. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines on the stability testing of new drug substances and products. Manufacturers must demonstrate that the use of ethyl propanoate as a stabilizer does not compromise the safety, efficacy, or quality of the active ingredients throughout the product's shelf life.
In cosmetic products, the use of ethyl propanoate is regulated by the FDA in the US and the European Commission in the EU. The Cosmetic Ingredient Review (CIR) Expert Panel has evaluated the safety of ethyl propanoate for use in cosmetic formulations and concluded that it is safe in the present practices of use and concentration.
Environmental regulations also play a role in the use of ethyl propanoate. The compound is classified as a volatile organic compound (VOC) and may be subject to emission control regulations in certain jurisdictions. Manufacturers must consider these environmental aspects when incorporating ethyl propanoate into their products or processes.
As the application of ethyl propanoate for extending the stability of active ingredients is relatively novel, regulatory bodies may require additional safety and efficacy data. Companies seeking to utilize this technology should engage with regulatory authorities early in the development process to ensure compliance with all applicable regulations and to address any potential concerns regarding its use as a stabilizer.
In the European Union, the European Food Safety Authority (EFSA) oversees the safety assessment of food additives. Ethyl propanoate is listed in Regulation (EC) No 1334/2008 as a flavoring substance that can be used in food products. For its use as a stabilizer in other applications, manufacturers must comply with the relevant EU regulations, such as the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation for chemical substances.
The regulatory landscape for ethyl propanoate in pharmaceutical applications is more complex. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines on the stability testing of new drug substances and products. Manufacturers must demonstrate that the use of ethyl propanoate as a stabilizer does not compromise the safety, efficacy, or quality of the active ingredients throughout the product's shelf life.
In cosmetic products, the use of ethyl propanoate is regulated by the FDA in the US and the European Commission in the EU. The Cosmetic Ingredient Review (CIR) Expert Panel has evaluated the safety of ethyl propanoate for use in cosmetic formulations and concluded that it is safe in the present practices of use and concentration.
Environmental regulations also play a role in the use of ethyl propanoate. The compound is classified as a volatile organic compound (VOC) and may be subject to emission control regulations in certain jurisdictions. Manufacturers must consider these environmental aspects when incorporating ethyl propanoate into their products or processes.
As the application of ethyl propanoate for extending the stability of active ingredients is relatively novel, regulatory bodies may require additional safety and efficacy data. Companies seeking to utilize this technology should engage with regulatory authorities early in the development process to ensure compliance with all applicable regulations and to address any potential concerns regarding its use as a stabilizer.
Environmental Impact Assessment
The use of ethyl propanoate as a stabilizer for active ingredients has potential environmental implications that warrant careful consideration. This ester compound, while effective in extending the shelf life of various formulations, may impact ecosystems if released into the environment in significant quantities.
Ethyl propanoate is generally considered to have low toxicity and is biodegradable, which mitigates some environmental concerns. However, its production and disposal still require attention to minimize ecological footprint. The synthesis of ethyl propanoate typically involves the reaction of ethanol with propionic acid or its derivatives, processes that may generate waste products and consume energy resources.
In aquatic environments, ethyl propanoate has the potential to affect water quality and aquatic life. Although it is soluble in water and expected to biodegrade rapidly, high concentrations could temporarily alter pH levels or dissolved oxygen content in water bodies. This could potentially stress sensitive aquatic organisms or disrupt local ecosystems if large quantities are discharged without proper treatment.
Atmospheric release of ethyl propanoate is another consideration. As a volatile organic compound (VOC), it can contribute to the formation of ground-level ozone when exposed to sunlight and nitrogen oxides. While its impact is generally considered less severe than many other VOCs, cumulative effects from industrial-scale use should not be overlooked in air quality assessments.
Soil contamination risks are relatively low due to the compound's volatility and biodegradability. However, repeated or large-scale spills could potentially affect soil microorganisms or plant life in the immediate vicinity. Proper handling and storage protocols are essential to prevent such incidents and protect soil ecosystems.
The environmental impact of ethyl propanoate must also be considered in the context of its role in extending the stability of active ingredients. By prolonging the shelf life of products, it may indirectly reduce waste generation and the need for frequent production cycles, potentially offsetting some of its direct environmental impacts through resource conservation.
In conclusion, while ethyl propanoate presents a relatively low environmental risk profile compared to many chemical stabilizers, its use should still be accompanied by responsible management practices. This includes optimizing production processes, implementing effective waste treatment systems, and ensuring proper handling throughout the product lifecycle. Ongoing monitoring and research into potential long-term ecological effects are advisable to ensure sustainable use of this compound in stabilizing active ingredients.
Ethyl propanoate is generally considered to have low toxicity and is biodegradable, which mitigates some environmental concerns. However, its production and disposal still require attention to minimize ecological footprint. The synthesis of ethyl propanoate typically involves the reaction of ethanol with propionic acid or its derivatives, processes that may generate waste products and consume energy resources.
In aquatic environments, ethyl propanoate has the potential to affect water quality and aquatic life. Although it is soluble in water and expected to biodegrade rapidly, high concentrations could temporarily alter pH levels or dissolved oxygen content in water bodies. This could potentially stress sensitive aquatic organisms or disrupt local ecosystems if large quantities are discharged without proper treatment.
Atmospheric release of ethyl propanoate is another consideration. As a volatile organic compound (VOC), it can contribute to the formation of ground-level ozone when exposed to sunlight and nitrogen oxides. While its impact is generally considered less severe than many other VOCs, cumulative effects from industrial-scale use should not be overlooked in air quality assessments.
Soil contamination risks are relatively low due to the compound's volatility and biodegradability. However, repeated or large-scale spills could potentially affect soil microorganisms or plant life in the immediate vicinity. Proper handling and storage protocols are essential to prevent such incidents and protect soil ecosystems.
The environmental impact of ethyl propanoate must also be considered in the context of its role in extending the stability of active ingredients. By prolonging the shelf life of products, it may indirectly reduce waste generation and the need for frequent production cycles, potentially offsetting some of its direct environmental impacts through resource conservation.
In conclusion, while ethyl propanoate presents a relatively low environmental risk profile compared to many chemical stabilizers, its use should still be accompanied by responsible management practices. This includes optimizing production processes, implementing effective waste treatment systems, and ensuring proper handling throughout the product lifecycle. Ongoing monitoring and research into potential long-term ecological effects are advisable to ensure sustainable use of this compound in stabilizing active ingredients.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!