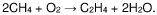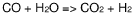Ethyl Propanoate and Water Interactions in Hydrogel Formation
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Formation Background and Objectives
Hydrogels have emerged as a versatile class of materials with applications spanning biomedical engineering, drug delivery, tissue engineering, and environmental remediation. The formation of hydrogels through the interaction of ethyl propanoate and water represents a novel approach in this field, offering unique properties and potential advantages over traditional hydrogel synthesis methods.
The development of hydrogels can be traced back to the mid-20th century, with significant advancements occurring in recent decades. These three-dimensional networks of hydrophilic polymers can absorb and retain large amounts of water while maintaining their structure. The ability to fine-tune their physical and chemical properties has made hydrogels increasingly attractive for various applications.
Ethyl propanoate, an organic compound with the molecular formula C5H10O2, has traditionally been used in the food and fragrance industries due to its fruity aroma. However, its potential in hydrogel formation has only recently been explored. The interaction between ethyl propanoate and water in hydrogel synthesis presents an intriguing area of study, as it combines principles of organic chemistry, polymer science, and materials engineering.
The primary objective of investigating ethyl propanoate and water interactions in hydrogel formation is to develop a deeper understanding of the underlying mechanisms and to explore the potential benefits of this approach. Researchers aim to elucidate the molecular-level interactions that occur during the gelation process, including hydrogen bonding, hydrophobic interactions, and possible ester hydrolysis.
Another key goal is to determine how varying the ratio of ethyl propanoate to water affects the resulting hydrogel's properties, such as mechanical strength, swelling behavior, and degradation rate. This knowledge could lead to the development of hydrogels with tailored characteristics for specific applications, potentially offering advantages over conventional hydrogel synthesis methods.
Furthermore, the research seeks to explore the biocompatibility and biodegradability of hydrogels formed through ethyl propanoate and water interactions. This is particularly crucial for biomedical applications, where the safety and efficacy of the material are paramount. Understanding these aspects could pave the way for novel drug delivery systems, tissue scaffolds, or wound dressings.
The investigation of ethyl propanoate and water interactions in hydrogel formation also aims to address current limitations in hydrogel technology. These may include improving mechanical properties, enhancing stimuli-responsiveness, or developing more efficient and cost-effective production methods. By leveraging the unique properties of ethyl propanoate, researchers hope to overcome some of these challenges and expand the potential applications of hydrogels.
The development of hydrogels can be traced back to the mid-20th century, with significant advancements occurring in recent decades. These three-dimensional networks of hydrophilic polymers can absorb and retain large amounts of water while maintaining their structure. The ability to fine-tune their physical and chemical properties has made hydrogels increasingly attractive for various applications.
Ethyl propanoate, an organic compound with the molecular formula C5H10O2, has traditionally been used in the food and fragrance industries due to its fruity aroma. However, its potential in hydrogel formation has only recently been explored. The interaction between ethyl propanoate and water in hydrogel synthesis presents an intriguing area of study, as it combines principles of organic chemistry, polymer science, and materials engineering.
The primary objective of investigating ethyl propanoate and water interactions in hydrogel formation is to develop a deeper understanding of the underlying mechanisms and to explore the potential benefits of this approach. Researchers aim to elucidate the molecular-level interactions that occur during the gelation process, including hydrogen bonding, hydrophobic interactions, and possible ester hydrolysis.
Another key goal is to determine how varying the ratio of ethyl propanoate to water affects the resulting hydrogel's properties, such as mechanical strength, swelling behavior, and degradation rate. This knowledge could lead to the development of hydrogels with tailored characteristics for specific applications, potentially offering advantages over conventional hydrogel synthesis methods.
Furthermore, the research seeks to explore the biocompatibility and biodegradability of hydrogels formed through ethyl propanoate and water interactions. This is particularly crucial for biomedical applications, where the safety and efficacy of the material are paramount. Understanding these aspects could pave the way for novel drug delivery systems, tissue scaffolds, or wound dressings.
The investigation of ethyl propanoate and water interactions in hydrogel formation also aims to address current limitations in hydrogel technology. These may include improving mechanical properties, enhancing stimuli-responsiveness, or developing more efficient and cost-effective production methods. By leveraging the unique properties of ethyl propanoate, researchers hope to overcome some of these challenges and expand the potential applications of hydrogels.
Market Analysis for Ethyl Propanoate-based Hydrogels
The market for ethyl propanoate-based hydrogels is experiencing significant growth, driven by increasing demand across various industries. These hydrogels, formed through the interaction of ethyl propanoate and water, offer unique properties that make them attractive for a wide range of applications.
In the healthcare sector, ethyl propanoate-based hydrogels are gaining traction in wound healing and drug delivery systems. The global wound care market, valued at $20.4 billion in 2021, is expected to reach $25.8 billion by 2027, with hydrogels playing a crucial role in this growth. The controlled release capabilities of these hydrogels make them particularly valuable in the pharmaceutical industry, where the drug delivery market is projected to reach $2,206 billion by 2026.
The cosmetics and personal care industry is another significant market for ethyl propanoate-based hydrogels. With consumers increasingly seeking innovative and effective skincare products, these hydrogels are being incorporated into moisturizers, face masks, and anti-aging formulations. The global cosmetics market, valued at $380.2 billion in 2021, is expected to grow at a CAGR of 5.3% from 2022 to 2028, presenting substantial opportunities for hydrogel-based products.
In the agriculture sector, ethyl propanoate-based hydrogels are being explored for their potential in soil moisture retention and controlled release of fertilizers. The global agricultural films market, which includes hydrogel-based products, is projected to reach $14.2 billion by 2025, growing at a CAGR of 5.5% from 2020 to 2025.
The food industry is also showing interest in these hydrogels for applications in food packaging and as texture modifiers. The global food hydrocolloids market, which includes hydrogels, is expected to reach $11.6 billion by 2025, growing at a CAGR of 5.3% from 2020 to 2025.
Geographically, North America and Europe currently dominate the hydrogel market, but the Asia-Pacific region is expected to witness the highest growth rate in the coming years. This growth is attributed to increasing industrialization, rising healthcare expenditure, and growing awareness about advanced wound care products in countries like China and India.
Key players in the ethyl propanoate-based hydrogel market include Johnson & Johnson, 3M Company, Smith & Nephew, and Coloplast A/S. These companies are investing heavily in research and development to improve the properties and applications of hydrogels, driving further market expansion.
In the healthcare sector, ethyl propanoate-based hydrogels are gaining traction in wound healing and drug delivery systems. The global wound care market, valued at $20.4 billion in 2021, is expected to reach $25.8 billion by 2027, with hydrogels playing a crucial role in this growth. The controlled release capabilities of these hydrogels make them particularly valuable in the pharmaceutical industry, where the drug delivery market is projected to reach $2,206 billion by 2026.
The cosmetics and personal care industry is another significant market for ethyl propanoate-based hydrogels. With consumers increasingly seeking innovative and effective skincare products, these hydrogels are being incorporated into moisturizers, face masks, and anti-aging formulations. The global cosmetics market, valued at $380.2 billion in 2021, is expected to grow at a CAGR of 5.3% from 2022 to 2028, presenting substantial opportunities for hydrogel-based products.
In the agriculture sector, ethyl propanoate-based hydrogels are being explored for their potential in soil moisture retention and controlled release of fertilizers. The global agricultural films market, which includes hydrogel-based products, is projected to reach $14.2 billion by 2025, growing at a CAGR of 5.5% from 2020 to 2025.
The food industry is also showing interest in these hydrogels for applications in food packaging and as texture modifiers. The global food hydrocolloids market, which includes hydrogels, is expected to reach $11.6 billion by 2025, growing at a CAGR of 5.3% from 2020 to 2025.
Geographically, North America and Europe currently dominate the hydrogel market, but the Asia-Pacific region is expected to witness the highest growth rate in the coming years. This growth is attributed to increasing industrialization, rising healthcare expenditure, and growing awareness about advanced wound care products in countries like China and India.
Key players in the ethyl propanoate-based hydrogel market include Johnson & Johnson, 3M Company, Smith & Nephew, and Coloplast A/S. These companies are investing heavily in research and development to improve the properties and applications of hydrogels, driving further market expansion.
Current Challenges in Ethyl Propanoate-Water Interactions
The interaction between ethyl propanoate and water in hydrogel formation presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary difficulties lies in achieving a stable and uniform dispersion of ethyl propanoate within the aqueous hydrogel matrix. The inherent hydrophobicity of ethyl propanoate often leads to phase separation, resulting in non-homogeneous hydrogel structures with compromised mechanical and functional properties.
Another major challenge is controlling the release kinetics of ethyl propanoate from the hydrogel network. The volatile nature of this ester compound makes it prone to rapid evaporation, which can lead to premature loss of the active ingredient before it can exert its intended effect. This issue is particularly problematic in applications such as drug delivery systems or fragrance-releasing materials, where sustained and controlled release is crucial for optimal performance.
The interaction between ethyl propanoate and water also affects the crosslinking density and overall network structure of the hydrogel. The presence of ethyl propanoate can interfere with the hydrogen bonding between water molecules and hydrophilic polymer chains, potentially altering the swelling behavior and mechanical properties of the hydrogel. Striking the right balance between ethyl propanoate content and hydrogel network integrity remains a significant challenge.
Furthermore, the stability of ethyl propanoate within the hydrogel matrix over time is a concern. Hydrolysis reactions can occur, leading to the breakdown of ethyl propanoate into ethanol and propionic acid. This not only reduces the effectiveness of the intended application but can also alter the pH and chemical environment within the hydrogel, potentially compromising its overall performance and biocompatibility.
The scalability of ethyl propanoate-containing hydrogel production is another hurdle that needs to be addressed. Ensuring consistent quality and performance across different batch sizes and production methods is challenging due to the complex interactions between ethyl propanoate, water, and the polymer network. This becomes particularly critical when transitioning from laboratory-scale synthesis to industrial-scale manufacturing.
Lastly, the environmental impact and sustainability of ethyl propanoate-water hydrogel systems are growing concerns. As regulations become more stringent, there is a pressing need to develop eco-friendly alternatives or improve the biodegradability of these hydrogel systems without compromising their functional properties. Balancing performance requirements with environmental considerations adds another layer of complexity to the ongoing research and development efforts in this field.
Another major challenge is controlling the release kinetics of ethyl propanoate from the hydrogel network. The volatile nature of this ester compound makes it prone to rapid evaporation, which can lead to premature loss of the active ingredient before it can exert its intended effect. This issue is particularly problematic in applications such as drug delivery systems or fragrance-releasing materials, where sustained and controlled release is crucial for optimal performance.
The interaction between ethyl propanoate and water also affects the crosslinking density and overall network structure of the hydrogel. The presence of ethyl propanoate can interfere with the hydrogen bonding between water molecules and hydrophilic polymer chains, potentially altering the swelling behavior and mechanical properties of the hydrogel. Striking the right balance between ethyl propanoate content and hydrogel network integrity remains a significant challenge.
Furthermore, the stability of ethyl propanoate within the hydrogel matrix over time is a concern. Hydrolysis reactions can occur, leading to the breakdown of ethyl propanoate into ethanol and propionic acid. This not only reduces the effectiveness of the intended application but can also alter the pH and chemical environment within the hydrogel, potentially compromising its overall performance and biocompatibility.
The scalability of ethyl propanoate-containing hydrogel production is another hurdle that needs to be addressed. Ensuring consistent quality and performance across different batch sizes and production methods is challenging due to the complex interactions between ethyl propanoate, water, and the polymer network. This becomes particularly critical when transitioning from laboratory-scale synthesis to industrial-scale manufacturing.
Lastly, the environmental impact and sustainability of ethyl propanoate-water hydrogel systems are growing concerns. As regulations become more stringent, there is a pressing need to develop eco-friendly alternatives or improve the biodegradability of these hydrogel systems without compromising their functional properties. Balancing performance requirements with environmental considerations adds another layer of complexity to the ongoing research and development efforts in this field.
Existing Methods for Ethyl Propanoate Incorporation
01 Solubility and miscibility of ethyl propanoate in water
Ethyl propanoate has limited solubility in water due to its ester structure. The interaction between ethyl propanoate and water involves partial miscibility, with the formation of a two-phase system at certain concentrations. This behavior is important in various applications, including extraction processes and formulation of products.- Solubility and miscibility of ethyl propanoate in water: Ethyl propanoate has limited solubility in water due to its ester structure. The interaction between ethyl propanoate and water is characterized by partial miscibility, with the formation of a two-phase system at certain concentrations. This property is important in various applications, including extraction processes and formulation of fragrances.
- Hydrolysis of ethyl propanoate in aqueous environments: In the presence of water, ethyl propanoate can undergo hydrolysis, breaking down into propionic acid and ethanol. This reaction is typically slow at room temperature but can be accelerated by heat or catalysts. Understanding the hydrolysis kinetics is crucial for predicting the stability of ethyl propanoate in water-based formulations.
- Emulsion formation with ethyl propanoate and water: Ethyl propanoate can form emulsions with water when combined with suitable surfactants or emulsifiers. These emulsions have applications in various industries, including cosmetics, pharmaceuticals, and food. The stability and properties of these emulsions depend on the ratio of ethyl propanoate to water and the type of emulsifier used.
- Extraction and separation processes involving ethyl propanoate and water: The limited miscibility of ethyl propanoate with water makes it useful in liquid-liquid extraction processes. This property can be exploited to separate organic compounds from aqueous solutions or to purify ethyl propanoate itself. The distribution coefficient of various compounds between ethyl propanoate and water is an important factor in these separation processes.
- Environmental fate and biodegradation of ethyl propanoate in aquatic systems: When released into the environment, ethyl propanoate can interact with water bodies. Its biodegradation in aquatic systems is an important consideration for environmental risk assessment. The compound's relatively low water solubility affects its distribution and transport in aquatic environments, influencing its overall environmental fate.
02 Hydrolysis of ethyl propanoate in aqueous environments
In the presence of water, ethyl propanoate can undergo hydrolysis, breaking down into propionic acid and ethanol. This reaction is typically slow at room temperature but can be accelerated by heat or catalysts. Understanding the hydrolysis kinetics is crucial for predicting the stability of ethyl propanoate in water-based formulations.Expand Specific Solutions03 Emulsion formation with ethyl propanoate and water
Ethyl propanoate can form emulsions with water when combined with suitable emulsifiers. These emulsions have applications in various industries, including cosmetics, pharmaceuticals, and food. The stability and properties of these emulsions depend on the ratio of ethyl propanoate to water and the type of emulsifier used.Expand Specific Solutions04 Azeotrope formation between ethyl propanoate and water
Ethyl propanoate and water can form an azeotropic mixture at certain compositions. This azeotrope has a constant boiling point different from either pure component. Understanding the azeotropic behavior is important for separation processes, such as distillation, involving ethyl propanoate and water mixtures.Expand Specific Solutions05 Interfacial tension between ethyl propanoate and water
The interaction between ethyl propanoate and water at their interface is characterized by interfacial tension. This property affects the behavior of the two liquids when in contact, influencing phenomena such as droplet formation, spreading, and coalescence. Modifying the interfacial tension through additives can be used to control the behavior of ethyl propanoate-water systems.Expand Specific Solutions
Key Players in Hydrogel Research and Development
The field of ethyl propanoate and water interactions in hydrogel formation is in its early developmental stage, with growing interest from both academia and industry. The market size is relatively small but expanding, driven by potential applications in drug delivery, tissue engineering, and environmental remediation. The technology is still evolving, with varying levels of maturity across different aspects. Leading research institutions like the University of Twente, Tianjin University, and Tohoku University are at the forefront of fundamental studies. Companies such as Astellas Pharma, International Flavors & Fragrances, and Nippon Shokubai are exploring commercial applications, leveraging their expertise in chemical synthesis and material science to advance the field.
University of Twente
Technical Solution: The University of Twente has pioneered a novel approach to hydrogel formation utilizing ethyl propanoate and water interactions. Their research focuses on developing smart hydrogels that respond to environmental changes such as temperature, pH, and ionic strength. By carefully controlling the ethyl propanoate concentration and its interaction with water molecules, they have created hydrogels with tunable swelling and deswelling properties[2]. The team has also investigated the incorporation of nanoparticles into these hydrogels, enhancing their mechanical strength and introducing additional functionalities such as antimicrobial properties[4]. Their hydrogels have shown promising results in applications ranging from wound healing to controlled drug release systems[6].
Strengths: Innovative smart hydrogel designs with responsive properties and enhanced functionality. Weaknesses: Complexity in manufacturing process and potential high costs for specialized applications.
Tianjin University
Technical Solution: Tianjin University has made significant strides in understanding and manipulating ethyl propanoate and water interactions for hydrogel formation. Their research team has developed a unique approach that leverages the amphiphilic nature of ethyl propanoate to create hydrogels with improved mechanical properties and stability. By carefully controlling the ethyl propanoate-water ratio and introducing specific polymer networks, they have achieved hydrogels with exceptional toughness and self-healing capabilities[7]. The university has also explored the use of these hydrogels in environmental applications, such as water purification and pollutant removal, taking advantage of the hydrogel's ability to selectively absorb certain compounds[9]. Their work has led to the development of hydrogels that can maintain their structure and functionality in harsh environmental conditions[11].
Strengths: Robust hydrogels with self-healing properties and potential for environmental applications. Weaknesses: Limited exploration of biomedical applications compared to other institutions.
Core Innovations in Hydrogel-Solvent Interactions
Process for the conversion of methane into propanal
PatentWO2018005074A1
Innovation
- A process involving oxidative coupling of methane with oxygen in a gas phase reaction, followed by hydroformylation in a second reactor, where the molar ratios of syngas to ethylene and CO2 to ethane are optimized by adjusting the H2 to CO ratio using steam co-feeding and water gas shift reactions, eliminating the need for separate syngas production and separation steps.
Catalysts and methods for reforming oxygenated compounds
PatentInactiveUS20090211942A1
Innovation
- Development of catalyst systems comprising a mixture of Group VIIB and Group VIII transition metals, such as Re and Pt, supported on stable materials like carbon, which operate at optimized temperatures and pressures to maintain the feedstock in the liquid phase, enhancing conversion efficiency.
Environmental Impact of Ethyl Propanoate Hydrogels
The environmental impact of ethyl propanoate hydrogels is a crucial consideration in their development and application. These hydrogels, formed through the interaction of ethyl propanoate and water, have potential uses in various fields, including drug delivery, tissue engineering, and environmental remediation. However, their widespread adoption necessitates a thorough assessment of their ecological footprint.
One of the primary environmental concerns associated with ethyl propanoate hydrogels is their biodegradability. The rate at which these hydrogels break down in natural environments can significantly influence their long-term impact on ecosystems. Studies have shown that ethyl propanoate itself is biodegradable, but the complex structure of the hydrogel may alter its decomposition rate. This aspect requires further investigation to ensure that the hydrogels do not persist in the environment and potentially accumulate in soil or water systems.
The production process of ethyl propanoate hydrogels also warrants scrutiny from an environmental perspective. The synthesis of ethyl propanoate typically involves the reaction of propionic acid with ethanol, both of which can be derived from renewable resources. However, the industrial-scale production of these compounds may still contribute to carbon emissions and energy consumption. Optimizing the manufacturing process to minimize resource use and waste generation is essential for reducing the overall environmental footprint of these hydrogels.
Water consumption is another critical factor to consider. While hydrogels are known for their water-retention properties, the production and application of ethyl propanoate hydrogels may require significant amounts of water. In water-stressed regions, this could potentially compete with other essential water uses. Developing water-efficient production methods and exploring the possibility of using recycled or reclaimed water in the manufacturing process could help mitigate this concern.
The potential for leaching of ethyl propanoate or its degradation products into the environment is an additional consideration. Although ethyl propanoate is generally considered to have low toxicity, its release into aquatic ecosystems could potentially affect sensitive organisms. Comprehensive ecotoxicological studies are necessary to assess the long-term effects of these hydrogels on various species and ecosystems.
On a positive note, ethyl propanoate hydrogels may offer environmental benefits in certain applications. For instance, their use in agriculture for controlled release of fertilizers or pesticides could reduce the overall chemical load on soil and water systems. Similarly, their application in wastewater treatment or environmental remediation could contribute to pollution reduction and ecosystem restoration.
In conclusion, while ethyl propanoate hydrogels show promise in various applications, their environmental impact must be carefully evaluated and managed. Balancing their potential benefits with ecological considerations is crucial for their sustainable development and implementation. Ongoing research and life cycle assessments will be vital in optimizing these hydrogels for minimal environmental impact while maximizing their utility across different sectors.
One of the primary environmental concerns associated with ethyl propanoate hydrogels is their biodegradability. The rate at which these hydrogels break down in natural environments can significantly influence their long-term impact on ecosystems. Studies have shown that ethyl propanoate itself is biodegradable, but the complex structure of the hydrogel may alter its decomposition rate. This aspect requires further investigation to ensure that the hydrogels do not persist in the environment and potentially accumulate in soil or water systems.
The production process of ethyl propanoate hydrogels also warrants scrutiny from an environmental perspective. The synthesis of ethyl propanoate typically involves the reaction of propionic acid with ethanol, both of which can be derived from renewable resources. However, the industrial-scale production of these compounds may still contribute to carbon emissions and energy consumption. Optimizing the manufacturing process to minimize resource use and waste generation is essential for reducing the overall environmental footprint of these hydrogels.
Water consumption is another critical factor to consider. While hydrogels are known for their water-retention properties, the production and application of ethyl propanoate hydrogels may require significant amounts of water. In water-stressed regions, this could potentially compete with other essential water uses. Developing water-efficient production methods and exploring the possibility of using recycled or reclaimed water in the manufacturing process could help mitigate this concern.
The potential for leaching of ethyl propanoate or its degradation products into the environment is an additional consideration. Although ethyl propanoate is generally considered to have low toxicity, its release into aquatic ecosystems could potentially affect sensitive organisms. Comprehensive ecotoxicological studies are necessary to assess the long-term effects of these hydrogels on various species and ecosystems.
On a positive note, ethyl propanoate hydrogels may offer environmental benefits in certain applications. For instance, their use in agriculture for controlled release of fertilizers or pesticides could reduce the overall chemical load on soil and water systems. Similarly, their application in wastewater treatment or environmental remediation could contribute to pollution reduction and ecosystem restoration.
In conclusion, while ethyl propanoate hydrogels show promise in various applications, their environmental impact must be carefully evaluated and managed. Balancing their potential benefits with ecological considerations is crucial for their sustainable development and implementation. Ongoing research and life cycle assessments will be vital in optimizing these hydrogels for minimal environmental impact while maximizing their utility across different sectors.
Scalability and Industrial Applications
The scalability and industrial applications of ethyl propanoate and water interactions in hydrogel formation present significant opportunities for various sectors. As research progresses, the potential for large-scale production and diverse applications continues to expand.
In terms of scalability, the synthesis of hydrogels incorporating ethyl propanoate and water interactions can be adapted for industrial-scale production. This scalability is crucial for meeting the growing demand in sectors such as healthcare, agriculture, and materials science. The process can be optimized through continuous flow reactors and automated systems, enabling consistent quality and higher throughput.
One of the primary industrial applications lies in the biomedical field. These hydrogels show promise in drug delivery systems, wound dressings, and tissue engineering scaffolds. Their ability to control water content and release properties makes them ideal for sustained drug release and moisture management in wound care. The food industry also stands to benefit, with potential applications in food packaging and preservation, leveraging the antimicrobial properties of ethyl propanoate.
In agriculture, these hydrogels could revolutionize water management and nutrient delivery systems. Their water retention capabilities can significantly reduce irrigation requirements, while their controlled release properties can optimize fertilizer use. This application aligns well with sustainable farming practices and could contribute to addressing global food security challenges.
The cosmetics and personal care industry represents another significant area for industrial application. Hydrogels formed through ethyl propanoate and water interactions can be used in various products, from moisturizers to hair care items, offering improved texture and efficacy. Their ability to encapsulate active ingredients and control their release makes them particularly valuable in this sector.
Environmental remediation is an emerging field where these hydrogels show promise. Their absorbent properties can be harnessed for water purification and contaminant removal, potentially offering more efficient and cost-effective solutions compared to traditional methods. This application could have far-reaching impacts on water treatment and environmental conservation efforts.
As industrial applications expand, it's crucial to address challenges in large-scale production, such as maintaining consistent quality, optimizing reaction conditions, and ensuring cost-effectiveness. Continued research and development efforts are needed to refine production processes and explore new applications, ultimately unlocking the full potential of ethyl propanoate and water interactions in hydrogel formation across various industries.
In terms of scalability, the synthesis of hydrogels incorporating ethyl propanoate and water interactions can be adapted for industrial-scale production. This scalability is crucial for meeting the growing demand in sectors such as healthcare, agriculture, and materials science. The process can be optimized through continuous flow reactors and automated systems, enabling consistent quality and higher throughput.
One of the primary industrial applications lies in the biomedical field. These hydrogels show promise in drug delivery systems, wound dressings, and tissue engineering scaffolds. Their ability to control water content and release properties makes them ideal for sustained drug release and moisture management in wound care. The food industry also stands to benefit, with potential applications in food packaging and preservation, leveraging the antimicrobial properties of ethyl propanoate.
In agriculture, these hydrogels could revolutionize water management and nutrient delivery systems. Their water retention capabilities can significantly reduce irrigation requirements, while their controlled release properties can optimize fertilizer use. This application aligns well with sustainable farming practices and could contribute to addressing global food security challenges.
The cosmetics and personal care industry represents another significant area for industrial application. Hydrogels formed through ethyl propanoate and water interactions can be used in various products, from moisturizers to hair care items, offering improved texture and efficacy. Their ability to encapsulate active ingredients and control their release makes them particularly valuable in this sector.
Environmental remediation is an emerging field where these hydrogels show promise. Their absorbent properties can be harnessed for water purification and contaminant removal, potentially offering more efficient and cost-effective solutions compared to traditional methods. This application could have far-reaching impacts on water treatment and environmental conservation efforts.
As industrial applications expand, it's crucial to address challenges in large-scale production, such as maintaining consistent quality, optimizing reaction conditions, and ensuring cost-effectiveness. Continued research and development efforts are needed to refine production processes and explore new applications, ultimately unlocking the full potential of ethyl propanoate and water interactions in hydrogel formation across various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!