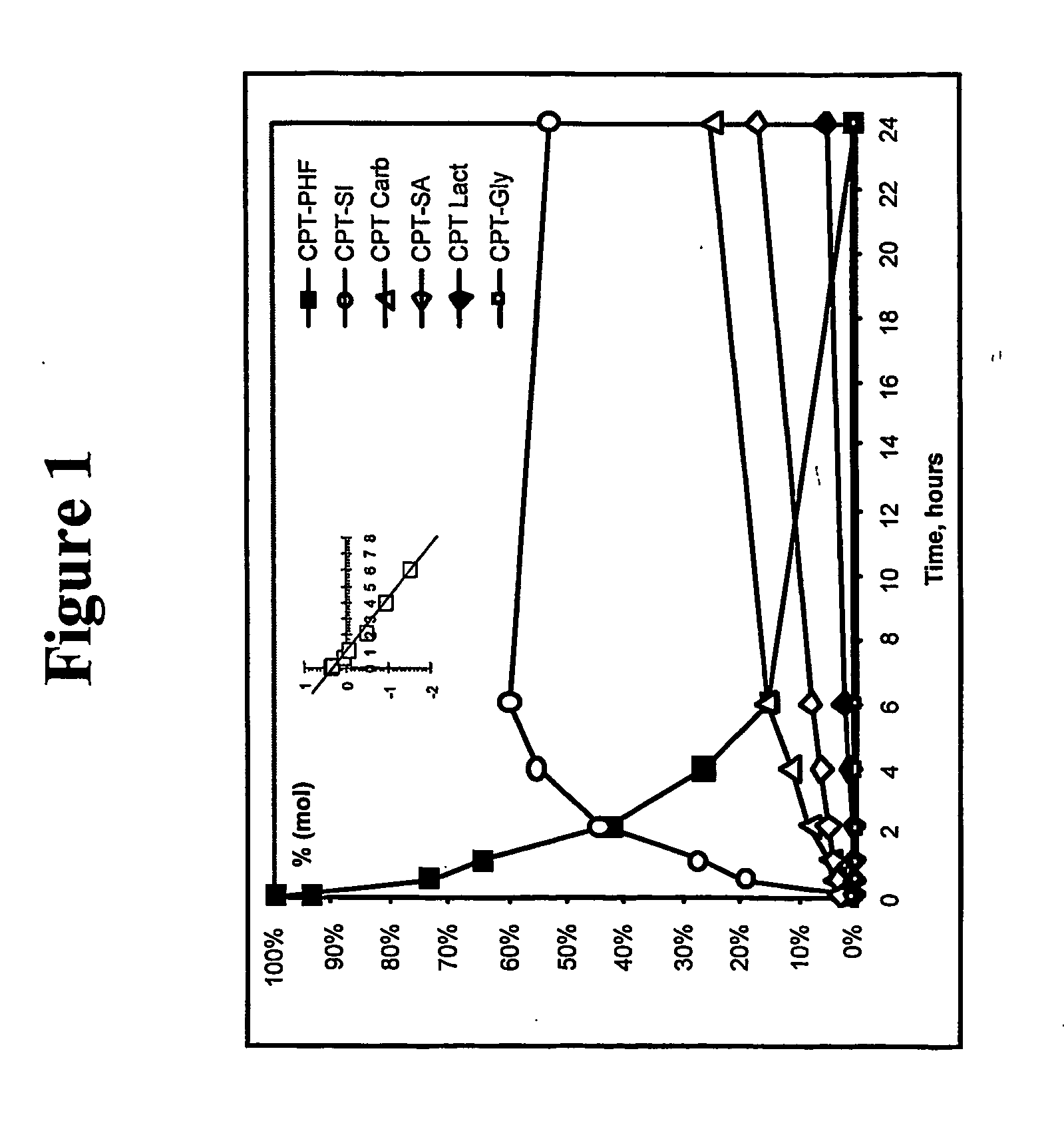
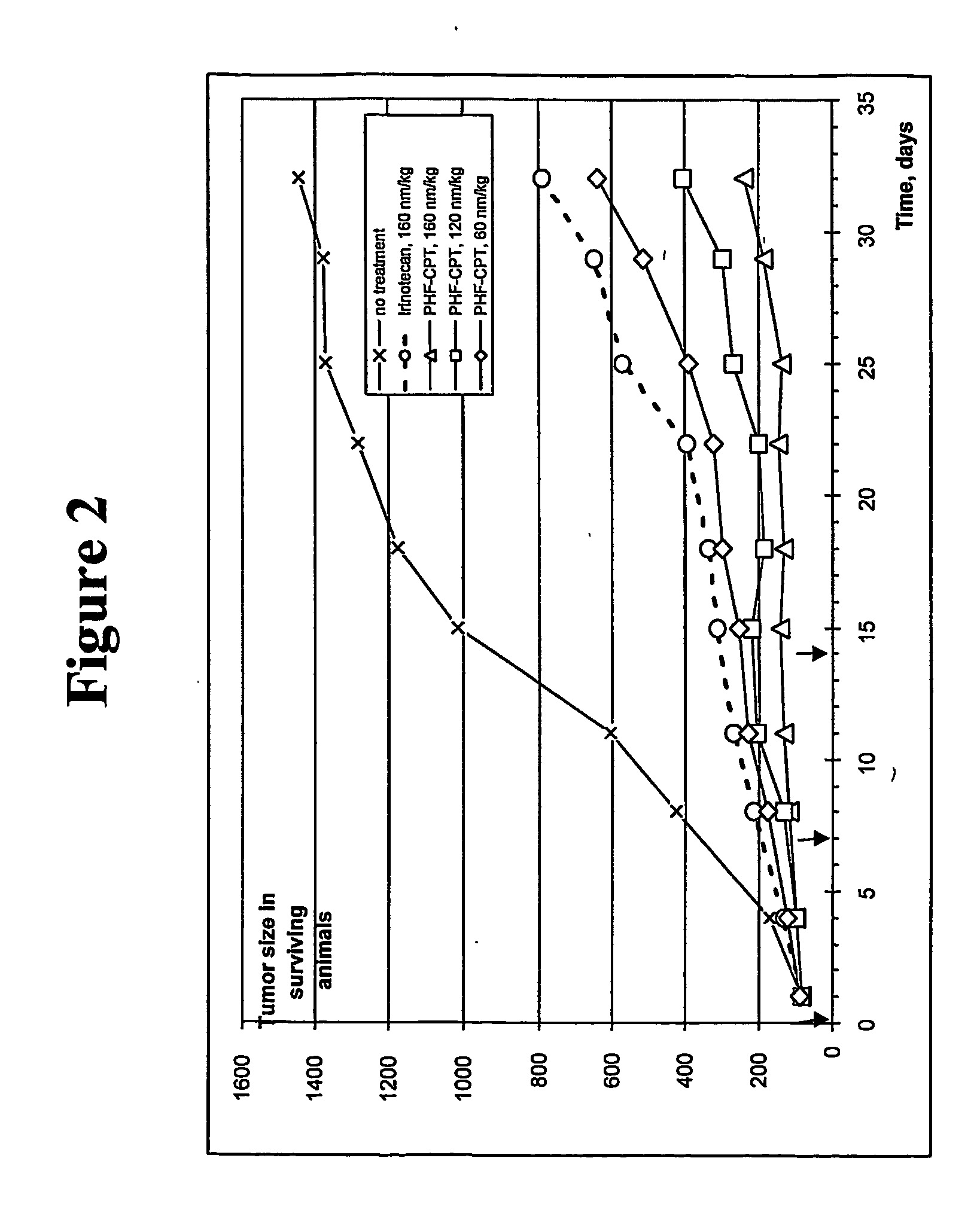
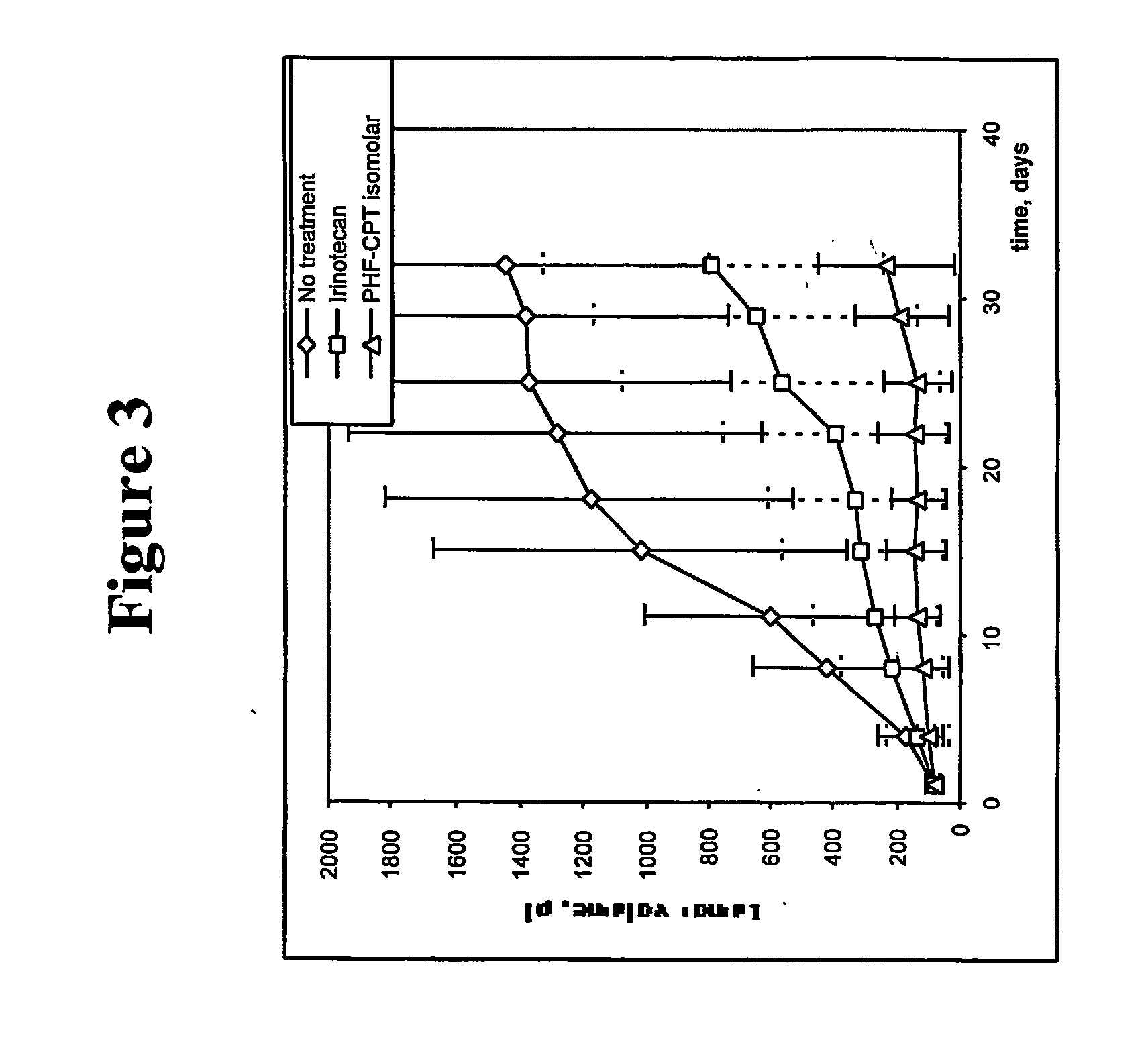
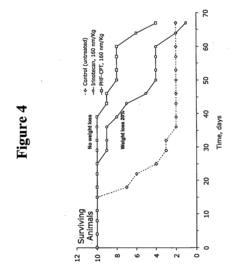
Biochemical Degradation Pathways of Ethyl Propanoate
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
Ethyl propanoate, also known as ethyl propionate, is an ester compound widely used in various industries, including food, cosmetics, and pharmaceuticals. The biochemical degradation of this compound has gained significant attention in recent years due to its environmental implications and potential applications in waste management and bioremediation.
The study of biochemical degradation pathways for ethyl propanoate is crucial for understanding its fate in natural environments and developing effective strategies for its removal from contaminated sites. This research area has evolved over the past few decades, with initial focus on identifying microorganisms capable of metabolizing the compound. Recent advancements in molecular biology and analytical techniques have enabled researchers to elucidate the specific enzymatic pathways involved in its breakdown.
The primary objective of this technical research report is to provide a comprehensive overview of the current state of knowledge regarding the biochemical degradation pathways of ethyl propanoate. This includes examining the key enzymes and metabolic intermediates involved in the process, as well as the environmental factors that influence degradation rates and efficiency.
Furthermore, this report aims to explore the potential applications of these degradation pathways in various fields. For instance, understanding these mechanisms can lead to the development of more efficient bioremediation strategies for contaminated soil and water. Additionally, insights into ethyl propanoate degradation may contribute to the design of novel biocatalysts for industrial processes or the improvement of existing biotechnological applications.
Another important aspect of this research is to investigate the microbial communities responsible for ethyl propanoate degradation in different environments. This includes identifying specific bacterial and fungal species that play key roles in the process, as well as studying their interactions and ecological dynamics. Such knowledge is essential for predicting the environmental fate of ethyl propanoate and developing targeted approaches for its management.
The technological evolution in this field has been marked by the integration of advanced analytical techniques, such as high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy, which have enabled researchers to identify and characterize metabolic intermediates with unprecedented precision. Additionally, the application of genomics and proteomics approaches has facilitated the discovery of novel enzymes and metabolic pathways involved in ethyl propanoate degradation.
In conclusion, this technical research report seeks to provide a thorough examination of the biochemical degradation pathways of ethyl propanoate, encompassing both fundamental scientific aspects and potential practical applications. By synthesizing current knowledge and identifying key research trends, this report aims to guide future investigations and technological developments in this important area of environmental biotechnology.
The study of biochemical degradation pathways for ethyl propanoate is crucial for understanding its fate in natural environments and developing effective strategies for its removal from contaminated sites. This research area has evolved over the past few decades, with initial focus on identifying microorganisms capable of metabolizing the compound. Recent advancements in molecular biology and analytical techniques have enabled researchers to elucidate the specific enzymatic pathways involved in its breakdown.
The primary objective of this technical research report is to provide a comprehensive overview of the current state of knowledge regarding the biochemical degradation pathways of ethyl propanoate. This includes examining the key enzymes and metabolic intermediates involved in the process, as well as the environmental factors that influence degradation rates and efficiency.
Furthermore, this report aims to explore the potential applications of these degradation pathways in various fields. For instance, understanding these mechanisms can lead to the development of more efficient bioremediation strategies for contaminated soil and water. Additionally, insights into ethyl propanoate degradation may contribute to the design of novel biocatalysts for industrial processes or the improvement of existing biotechnological applications.
Another important aspect of this research is to investigate the microbial communities responsible for ethyl propanoate degradation in different environments. This includes identifying specific bacterial and fungal species that play key roles in the process, as well as studying their interactions and ecological dynamics. Such knowledge is essential for predicting the environmental fate of ethyl propanoate and developing targeted approaches for its management.
The technological evolution in this field has been marked by the integration of advanced analytical techniques, such as high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy, which have enabled researchers to identify and characterize metabolic intermediates with unprecedented precision. Additionally, the application of genomics and proteomics approaches has facilitated the discovery of novel enzymes and metabolic pathways involved in ethyl propanoate degradation.
In conclusion, this technical research report seeks to provide a thorough examination of the biochemical degradation pathways of ethyl propanoate, encompassing both fundamental scientific aspects and potential practical applications. By synthesizing current knowledge and identifying key research trends, this report aims to guide future investigations and technological developments in this important area of environmental biotechnology.
Market Analysis
The market for biochemical degradation of ethyl propanoate is experiencing significant growth, driven by increasing environmental concerns and stringent regulations on waste management. This compound, commonly used as a solvent and flavoring agent, has applications across various industries, including food and beverage, pharmaceuticals, and cosmetics. As these sectors continue to expand, the demand for effective and environmentally friendly degradation methods for ethyl propanoate is also rising.
In the food and beverage industry, ethyl propanoate is widely used as a fruit flavoring, particularly for pineapple and strawberry flavors. The global flavors and fragrances market, which includes ethyl propanoate, is projected to grow steadily in the coming years. This growth is fueled by changing consumer preferences towards natural and organic products, driving the need for bio-based degradation methods for ethyl propanoate and similar compounds.
The pharmaceutical industry also contributes to the market demand for ethyl propanoate degradation. As the industry expands and faces increasing pressure to adopt sustainable practices, there is a growing need for efficient disposal methods of chemical waste, including ethyl propanoate. This trend is further reinforced by the implementation of stricter environmental regulations worldwide, compelling pharmaceutical companies to invest in advanced waste treatment technologies.
In the cosmetics and personal care sector, ethyl propanoate is used in various products as a solvent and fragrance ingredient. The global cosmetics market is experiencing robust growth, particularly in emerging economies. This expansion is accompanied by a heightened focus on sustainability and eco-friendly practices, creating opportunities for biochemical degradation solutions for compounds like ethyl propanoate.
The waste management industry is another key driver of the market for ethyl propanoate degradation. As landfill space becomes scarce and environmental regulations tighten, there is an increasing emphasis on developing efficient and sustainable waste treatment methods. Biochemical degradation offers a promising solution for handling organic compounds like ethyl propanoate, aligning with the growing trend towards circular economy principles.
Geographically, North America and Europe currently lead the market for biochemical degradation technologies, including those for ethyl propanoate. This is primarily due to stringent environmental regulations and high awareness of sustainable practices in these regions. However, the Asia-Pacific region is expected to witness the fastest growth in this market, driven by rapid industrialization, increasing environmental concerns, and government initiatives promoting sustainable development.
In the food and beverage industry, ethyl propanoate is widely used as a fruit flavoring, particularly for pineapple and strawberry flavors. The global flavors and fragrances market, which includes ethyl propanoate, is projected to grow steadily in the coming years. This growth is fueled by changing consumer preferences towards natural and organic products, driving the need for bio-based degradation methods for ethyl propanoate and similar compounds.
The pharmaceutical industry also contributes to the market demand for ethyl propanoate degradation. As the industry expands and faces increasing pressure to adopt sustainable practices, there is a growing need for efficient disposal methods of chemical waste, including ethyl propanoate. This trend is further reinforced by the implementation of stricter environmental regulations worldwide, compelling pharmaceutical companies to invest in advanced waste treatment technologies.
In the cosmetics and personal care sector, ethyl propanoate is used in various products as a solvent and fragrance ingredient. The global cosmetics market is experiencing robust growth, particularly in emerging economies. This expansion is accompanied by a heightened focus on sustainability and eco-friendly practices, creating opportunities for biochemical degradation solutions for compounds like ethyl propanoate.
The waste management industry is another key driver of the market for ethyl propanoate degradation. As landfill space becomes scarce and environmental regulations tighten, there is an increasing emphasis on developing efficient and sustainable waste treatment methods. Biochemical degradation offers a promising solution for handling organic compounds like ethyl propanoate, aligning with the growing trend towards circular economy principles.
Geographically, North America and Europe currently lead the market for biochemical degradation technologies, including those for ethyl propanoate. This is primarily due to stringent environmental regulations and high awareness of sustainable practices in these regions. However, the Asia-Pacific region is expected to witness the fastest growth in this market, driven by rapid industrialization, increasing environmental concerns, and government initiatives promoting sustainable development.
Technical Challenges
The biochemical degradation of ethyl propanoate presents several technical challenges that researchers and industry professionals must address. One of the primary obstacles is the complexity of the degradation pathways, which involve multiple enzymatic reactions and intermediate compounds. Understanding and controlling these intricate processes require advanced analytical techniques and sophisticated modeling approaches.
A significant challenge lies in identifying and characterizing the specific microorganisms and enzymes responsible for the degradation of ethyl propanoate. While some bacterial strains capable of metabolizing this ester have been identified, the complete enzymatic pathways and their regulation mechanisms remain unclear. This knowledge gap hinders the development of optimized biodegradation strategies and limits the potential for biotechnological applications.
Environmental factors pose another set of challenges in the biochemical degradation of ethyl propanoate. Temperature, pH, and oxygen availability significantly influence the efficiency of the degradation process. Maintaining optimal conditions for microbial activity in diverse environments, such as soil or wastewater treatment systems, can be technically demanding. Additionally, the presence of other organic compounds or pollutants may interfere with the degradation pathways, necessitating the development of robust and adaptable microbial consortia.
The formation and accumulation of intermediate metabolites during ethyl propanoate degradation present both analytical and environmental challenges. Some of these intermediates may be toxic or recalcitrant, potentially causing secondary pollution issues. Developing methods to detect, quantify, and manage these intermediates is crucial for ensuring complete mineralization of the compound and preventing unintended environmental impacts.
Scaling up laboratory-based degradation processes to industrial or environmental applications introduces additional technical hurdles. Factors such as mass transfer limitations, substrate availability, and microbial population dynamics become more pronounced at larger scales. Engineers must design bioreactors or in situ treatment systems that can maintain efficient degradation rates while addressing these scale-up challenges.
The genetic and metabolic engineering of microorganisms for enhanced ethyl propanoate degradation capabilities is a promising yet technically challenging approach. While genetic modification techniques have advanced significantly, optimizing the expression and activity of key enzymes involved in the degradation pathways remains complex. Balancing improved degradation efficiency with the overall metabolic stability and fitness of the engineered organisms is a delicate task that requires extensive research and testing.
Lastly, the development of reliable and cost-effective monitoring techniques for tracking the progress of ethyl propanoate degradation in real-time poses a significant technical challenge. Current analytical methods often involve time-consuming and expensive laboratory procedures. Developing rapid, on-site detection methods or biosensors specific to ethyl propanoate and its degradation products would greatly enhance the ability to monitor and optimize biodegradation processes in various applications.
A significant challenge lies in identifying and characterizing the specific microorganisms and enzymes responsible for the degradation of ethyl propanoate. While some bacterial strains capable of metabolizing this ester have been identified, the complete enzymatic pathways and their regulation mechanisms remain unclear. This knowledge gap hinders the development of optimized biodegradation strategies and limits the potential for biotechnological applications.
Environmental factors pose another set of challenges in the biochemical degradation of ethyl propanoate. Temperature, pH, and oxygen availability significantly influence the efficiency of the degradation process. Maintaining optimal conditions for microbial activity in diverse environments, such as soil or wastewater treatment systems, can be technically demanding. Additionally, the presence of other organic compounds or pollutants may interfere with the degradation pathways, necessitating the development of robust and adaptable microbial consortia.
The formation and accumulation of intermediate metabolites during ethyl propanoate degradation present both analytical and environmental challenges. Some of these intermediates may be toxic or recalcitrant, potentially causing secondary pollution issues. Developing methods to detect, quantify, and manage these intermediates is crucial for ensuring complete mineralization of the compound and preventing unintended environmental impacts.
Scaling up laboratory-based degradation processes to industrial or environmental applications introduces additional technical hurdles. Factors such as mass transfer limitations, substrate availability, and microbial population dynamics become more pronounced at larger scales. Engineers must design bioreactors or in situ treatment systems that can maintain efficient degradation rates while addressing these scale-up challenges.
The genetic and metabolic engineering of microorganisms for enhanced ethyl propanoate degradation capabilities is a promising yet technically challenging approach. While genetic modification techniques have advanced significantly, optimizing the expression and activity of key enzymes involved in the degradation pathways remains complex. Balancing improved degradation efficiency with the overall metabolic stability and fitness of the engineered organisms is a delicate task that requires extensive research and testing.
Lastly, the development of reliable and cost-effective monitoring techniques for tracking the progress of ethyl propanoate degradation in real-time poses a significant technical challenge. Current analytical methods often involve time-consuming and expensive laboratory procedures. Developing rapid, on-site detection methods or biosensors specific to ethyl propanoate and its degradation products would greatly enhance the ability to monitor and optimize biodegradation processes in various applications.
Current Solutions
01 Synthesis of ethyl propanoate
Ethyl propanoate can be synthesized through various methods, including the esterification of propionic acid with ethanol. This process typically involves catalysts and specific reaction conditions to achieve high yields and purity of the final product.- Synthesis of ethyl propanoate: Ethyl propanoate can be synthesized through various methods, including the esterification of propionic acid with ethanol. This process typically involves the use of catalysts and specific reaction conditions to optimize yield and purity. The synthesis can be carried out in both laboratory and industrial settings, with different approaches depending on the scale and desired product characteristics.
- Applications in flavor and fragrance industry: Ethyl propanoate is widely used in the flavor and fragrance industry due to its fruity, rum-like odor. It is commonly employed as a flavoring agent in food products and beverages, as well as in perfumes and cosmetics. The compound's pleasant aroma makes it a versatile ingredient in various consumer products, contributing to their overall sensory profile.
- Use in pharmaceutical formulations: Ethyl propanoate finds applications in the pharmaceutical industry, particularly in drug formulations and delivery systems. It can be used as a solvent or excipient in various pharmaceutical preparations, helping to improve drug solubility, stability, or bioavailability. The compound's properties make it suitable for incorporation into different dosage forms and drug delivery platforms.
- Industrial solvent and chemical intermediate: Ethyl propanoate serves as an important industrial solvent and chemical intermediate. It is used in the production of various chemicals, polymers, and materials. The compound's solvent properties make it useful in cleaning applications, paint formulations, and as a reaction medium for organic syntheses. Its reactivity also allows for its use as a building block in the synthesis of more complex molecules.
- Environmental and safety considerations: The use and handling of ethyl propanoate require consideration of environmental and safety aspects. This includes proper storage, handling, and disposal practices to minimize environmental impact and ensure worker safety. Research and development efforts focus on developing greener production methods, improving its biodegradability, and assessing its potential long-term effects on human health and the environment.
02 Applications in fragrance and flavor industry
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is employed in the creation of artificial fruit flavors, particularly for pineapple and strawberry scents, and is also used in perfumery to add sweet, ethereal notes to various compositions.Expand Specific Solutions03 Use as a solvent and intermediate
Ethyl propanoate serves as an important solvent in various industrial applications, including the production of paints, inks, and coatings. It is also used as an intermediate in the synthesis of other chemicals and pharmaceutical compounds.Expand Specific Solutions04 Production methods and process improvements
Research and development efforts focus on improving the production methods of ethyl propanoate, including the development of new catalysts, optimization of reaction conditions, and implementation of continuous flow processes to enhance efficiency and reduce environmental impact.Expand Specific Solutions05 Environmental and safety considerations
As ethyl propanoate is used in various industries, there is ongoing research into its environmental impact and safety profile. This includes studies on its biodegradability, toxicity, and potential alternatives for more sustainable production and use in different applications.Expand Specific Solutions
Key Industry Players
The biochemical degradation pathways of ethyl propanoate represent a niche area within the broader field of biotechnology and chemical engineering. The market is in its early development stage, with limited commercial applications but growing research interest. Key players include academic institutions like The Regents of the University of California and Jiangnan University, alongside industry leaders such as METabolic EXplorer SA and Genomatica, Inc. These organizations are at various stages of technological maturity, ranging from basic research to pilot-scale demonstrations. The market size remains relatively small, primarily driven by research funding and potential industrial applications in sustainable chemical production. As environmental concerns grow, this field may see increased attention and investment in the coming years.
The Regents of the University of California
Technical Solution: Researchers at the University of California have developed a novel approach to understanding and manipulating the biochemical degradation pathways of ethyl propanoate. Their work focuses on elucidating the microbial metabolic networks involved in ethyl propanoate breakdown using advanced genomics and metabolomics techniques. They have identified and characterized key enzymes in the degradation pathway, including esterases, alcohol dehydrogenases, and aldehyde dehydrogenases. Using synthetic biology tools, the team has engineered microbial strains with enhanced capabilities for ethyl propanoate degradation. They have also developed a machine learning model to predict degradation rates and pathways under various environmental conditions. This comprehensive understanding of the degradation process has led to the development of bioremediation strategies for ethyl propanoate contamination in soil and water systems [8][10].
Strengths: Fundamental understanding of degradation mechanisms, potential for tailored bioremediation solutions, integration of advanced computational methods. Weaknesses: Early stage of development, potential challenges in scaling up to industrial applications, regulatory hurdles for engineered microorganisms.
Genomatica, Inc.
Technical Solution: Genomatica has developed a bio-based process for producing propanoic acid, a precursor to ethyl propanoate, using engineered microorganisms. Their approach involves fermenting renewable feedstocks like sugar or glycerin to produce propanoic acid. The company has optimized the metabolic pathways of their proprietary microorganisms to enhance yield and productivity. They employ advanced bioreactor designs and continuous fermentation techniques to improve efficiency. Genomatica's process integrates downstream separation and purification steps, including distillation and esterification, to convert the propanoic acid to ethyl propanoate. This bio-based route offers a more sustainable alternative to traditional petrochemical-based production methods [1][3].
Strengths: Renewable feedstock usage, reduced carbon footprint, potential for cost-competitiveness with petrochemical routes. Weaknesses: Scaling challenges, feedstock price volatility, potential contamination risks in fermentation.
Core Innovations
Acyl amino acid production
PatentInactiveUS20180127791A1
Innovation
- Genetically modified microbial cells with specific mutations that reduce glutamate breakdown and enhance the production of acyl amino acids by increasing the activity of enzymes like glycine-N-acyl transferase and acyl-CoA synthetase, allowing for the conversion of amino and fatty acids into acyl amino acids with improved yield and purity.
Dual phase drug release system
PatentInactiveUS20070190018A1
Innovation
- The development of biodegradable, biocompatible polymer conjugates with succinamide-containing linkages, where modifiers are covalently attached to a carrier via amide bonds and linked through ester bonds, enabling dual-phase drug release and improved pharmacokinetics.
Environmental Impact
The biochemical degradation of ethyl propanoate has significant environmental implications, particularly in terms of its impact on ecosystems and potential contributions to atmospheric pollution. As this ester compound breaks down, it releases ethanol and propanoic acid, which can have varying effects on different environmental compartments.
In aquatic environments, the degradation of ethyl propanoate can lead to localized changes in pH levels, potentially affecting sensitive aquatic organisms. The release of ethanol may contribute to increased biological oxygen demand (BOD) in water bodies, temporarily depleting oxygen levels and impacting aquatic life. However, the relatively rapid biodegradation of ethanol by microorganisms typically limits long-term effects.
Soil ecosystems may experience short-term changes in microbial community structures as ethyl propanoate degrades. The released compounds can serve as carbon sources for certain soil microorganisms, potentially altering local nutrient cycles. While this process generally does not pose significant long-term risks to soil health, high concentrations in localized areas could temporarily affect soil pH and microbial activity.
In the atmosphere, the degradation of ethyl propanoate contributes to the formation of secondary organic aerosols (SOAs). These fine particulate matters can impact air quality and play a role in cloud formation processes. The breakdown products, particularly ethanol, may also participate in photochemical reactions, potentially contributing to the formation of ground-level ozone under certain conditions.
From a global perspective, the environmental impact of ethyl propanoate degradation is generally considered low due to its relatively rapid breakdown and the ubiquity of microbial degradation pathways in nature. However, in industrial settings or areas with high concentrations, monitoring and proper management are essential to mitigate potential localized effects on air and water quality.
The biodegradability of ethyl propanoate and its breakdown products aligns with principles of green chemistry, as it reduces the persistence of synthetic compounds in the environment. This characteristic makes it a preferable option in many applications compared to more persistent organic compounds. However, responsible use and disposal practices remain crucial to minimize any potential negative environmental impacts, particularly in sensitive ecosystems or areas prone to pollution accumulation.
In aquatic environments, the degradation of ethyl propanoate can lead to localized changes in pH levels, potentially affecting sensitive aquatic organisms. The release of ethanol may contribute to increased biological oxygen demand (BOD) in water bodies, temporarily depleting oxygen levels and impacting aquatic life. However, the relatively rapid biodegradation of ethanol by microorganisms typically limits long-term effects.
Soil ecosystems may experience short-term changes in microbial community structures as ethyl propanoate degrades. The released compounds can serve as carbon sources for certain soil microorganisms, potentially altering local nutrient cycles. While this process generally does not pose significant long-term risks to soil health, high concentrations in localized areas could temporarily affect soil pH and microbial activity.
In the atmosphere, the degradation of ethyl propanoate contributes to the formation of secondary organic aerosols (SOAs). These fine particulate matters can impact air quality and play a role in cloud formation processes. The breakdown products, particularly ethanol, may also participate in photochemical reactions, potentially contributing to the formation of ground-level ozone under certain conditions.
From a global perspective, the environmental impact of ethyl propanoate degradation is generally considered low due to its relatively rapid breakdown and the ubiquity of microbial degradation pathways in nature. However, in industrial settings or areas with high concentrations, monitoring and proper management are essential to mitigate potential localized effects on air and water quality.
The biodegradability of ethyl propanoate and its breakdown products aligns with principles of green chemistry, as it reduces the persistence of synthetic compounds in the environment. This characteristic makes it a preferable option in many applications compared to more persistent organic compounds. However, responsible use and disposal practices remain crucial to minimize any potential negative environmental impacts, particularly in sensitive ecosystems or areas prone to pollution accumulation.
Regulatory Framework
The regulatory framework surrounding the biochemical degradation of ethyl propanoate is complex and multifaceted, involving various governmental agencies and international bodies. At the forefront of this regulatory landscape is the Environmental Protection Agency (EPA) in the United States, which oversees the regulation of chemical substances under the Toxic Substances Control Act (TSCA). The EPA has established guidelines for the safe handling, storage, and disposal of ethyl propanoate and its degradation products.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation plays a crucial role in managing the risks associated with chemical substances, including ethyl propanoate. REACH requires manufacturers and importers to register their substances and provide safety data, ensuring a comprehensive understanding of the chemical's properties and potential environmental impacts.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards worldwide. Under GHS, ethyl propanoate is classified as a flammable liquid and is subject to specific labeling and safety data sheet requirements. This system facilitates international trade while promoting safety and environmental protection.
Regulatory bodies also focus on the environmental fate of ethyl propanoate and its degradation products. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing the biodegradability of chemicals, which are widely used to assess the environmental persistence of substances like ethyl propanoate. These tests help determine whether a chemical meets the criteria for being considered readily biodegradable.
In the context of waste management, regulations such as the Resource Conservation and Recovery Act (RCRA) in the United States govern the disposal of ethyl propanoate and its byproducts. Facilities handling these substances must comply with strict waste characterization, storage, and treatment requirements to prevent environmental contamination.
The regulatory framework also extends to occupational safety, with agencies like the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) setting exposure limits and safety protocols for workers handling ethyl propanoate and related compounds. These regulations aim to protect workers from potential health hazards associated with chemical exposure.
As research into the biochemical degradation pathways of ethyl propanoate progresses, regulatory frameworks are likely to evolve. Emerging scientific findings may lead to updates in risk assessments, exposure limits, and environmental guidelines. Regulatory agencies increasingly emphasize the importance of understanding degradation pathways in assessing the overall environmental impact of chemicals, potentially influencing future policy decisions and regulatory approaches.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation plays a crucial role in managing the risks associated with chemical substances, including ethyl propanoate. REACH requires manufacturers and importers to register their substances and provide safety data, ensuring a comprehensive understanding of the chemical's properties and potential environmental impacts.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards worldwide. Under GHS, ethyl propanoate is classified as a flammable liquid and is subject to specific labeling and safety data sheet requirements. This system facilitates international trade while promoting safety and environmental protection.
Regulatory bodies also focus on the environmental fate of ethyl propanoate and its degradation products. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing the biodegradability of chemicals, which are widely used to assess the environmental persistence of substances like ethyl propanoate. These tests help determine whether a chemical meets the criteria for being considered readily biodegradable.
In the context of waste management, regulations such as the Resource Conservation and Recovery Act (RCRA) in the United States govern the disposal of ethyl propanoate and its byproducts. Facilities handling these substances must comply with strict waste characterization, storage, and treatment requirements to prevent environmental contamination.
The regulatory framework also extends to occupational safety, with agencies like the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) setting exposure limits and safety protocols for workers handling ethyl propanoate and related compounds. These regulations aim to protect workers from potential health hazards associated with chemical exposure.
As research into the biochemical degradation pathways of ethyl propanoate progresses, regulatory frameworks are likely to evolve. Emerging scientific findings may lead to updates in risk assessments, exposure limits, and environmental guidelines. Regulatory agencies increasingly emphasize the importance of understanding degradation pathways in assessing the overall environmental impact of chemicals, potentially influencing future policy decisions and regulatory approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!