Ethyl Propanoate in Biomedical Adhesive Development
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate in Biomedical Adhesives: Background and Objectives
Ethyl propanoate, also known as ethyl propionate, has emerged as a promising compound in the development of biomedical adhesives. This ester, with its unique chemical properties, has garnered significant attention in the medical field due to its potential to enhance adhesive performance in biological environments. The evolution of biomedical adhesives has been driven by the increasing demand for minimally invasive surgical procedures and the need for more effective wound closure techniques.
The primary objective of researching ethyl propanoate in biomedical adhesive development is to create advanced adhesive formulations that exhibit superior biocompatibility, strong adhesion in wet environments, and controlled biodegradability. These characteristics are crucial for applications in tissue engineering, wound healing, and surgical interventions. The integration of ethyl propanoate into adhesive systems aims to address the limitations of current biomedical adhesives, such as poor wet adhesion and potential toxicity.
Historically, biomedical adhesives have progressed from simple natural adhesives to sophisticated synthetic formulations. The introduction of cyanoacrylates in the 1950s marked a significant milestone in this field. However, the search for improved adhesives with enhanced biocompatibility and performance has continued, leading to the exploration of novel compounds like ethyl propanoate.
The technological trajectory of biomedical adhesives incorporating ethyl propanoate is closely aligned with advancements in polymer science and biomaterials engineering. Researchers are investigating the compound's ability to modify the mechanical properties of adhesives, improve their wetting characteristics, and enhance their interaction with biological tissues. The goal is to develop adhesives that can form strong bonds under challenging physiological conditions while maintaining biocompatibility.
Current research efforts are focused on understanding the molecular interactions between ethyl propanoate and various polymer matrices. Scientists are exploring its role in modulating adhesive strength, setting time, and degradation profiles. Additionally, studies are being conducted to assess the compound's impact on cell adhesion and proliferation, which are critical factors in wound healing and tissue regeneration.
The development of ethyl propanoate-based biomedical adhesives is expected to have far-reaching implications in various medical applications. These include improved surgical sealants, advanced wound dressings, and novel drug delivery systems. The research aims to push the boundaries of current adhesive technologies, potentially revolutionizing minimally invasive surgical techniques and tissue engineering approaches.
The primary objective of researching ethyl propanoate in biomedical adhesive development is to create advanced adhesive formulations that exhibit superior biocompatibility, strong adhesion in wet environments, and controlled biodegradability. These characteristics are crucial for applications in tissue engineering, wound healing, and surgical interventions. The integration of ethyl propanoate into adhesive systems aims to address the limitations of current biomedical adhesives, such as poor wet adhesion and potential toxicity.
Historically, biomedical adhesives have progressed from simple natural adhesives to sophisticated synthetic formulations. The introduction of cyanoacrylates in the 1950s marked a significant milestone in this field. However, the search for improved adhesives with enhanced biocompatibility and performance has continued, leading to the exploration of novel compounds like ethyl propanoate.
The technological trajectory of biomedical adhesives incorporating ethyl propanoate is closely aligned with advancements in polymer science and biomaterials engineering. Researchers are investigating the compound's ability to modify the mechanical properties of adhesives, improve their wetting characteristics, and enhance their interaction with biological tissues. The goal is to develop adhesives that can form strong bonds under challenging physiological conditions while maintaining biocompatibility.
Current research efforts are focused on understanding the molecular interactions between ethyl propanoate and various polymer matrices. Scientists are exploring its role in modulating adhesive strength, setting time, and degradation profiles. Additionally, studies are being conducted to assess the compound's impact on cell adhesion and proliferation, which are critical factors in wound healing and tissue regeneration.
The development of ethyl propanoate-based biomedical adhesives is expected to have far-reaching implications in various medical applications. These include improved surgical sealants, advanced wound dressings, and novel drug delivery systems. The research aims to push the boundaries of current adhesive technologies, potentially revolutionizing minimally invasive surgical techniques and tissue engineering approaches.
Market Analysis for Biomedical Adhesive Applications
The biomedical adhesive market has been experiencing significant growth in recent years, driven by increasing demand in surgical procedures, wound closure, and medical device assembly. The global market for biomedical adhesives is projected to reach substantial value by 2027, with a compound annual growth rate (CAGR) exceeding 7% during the forecast period. This growth is attributed to the rising prevalence of chronic diseases, an aging population, and advancements in medical technologies.
Within this expanding market, ethyl propanoate has emerged as a promising component in biomedical adhesive development. Its potential applications span across various medical fields, including orthopedics, dentistry, and cardiovascular surgery. The demand for ethyl propanoate-based adhesives is particularly strong in wound closure and tissue engineering applications, where biocompatibility and controlled degradation are crucial factors.
The orthopedic segment is expected to hold a significant market share, driven by the increasing number of bone and joint surgeries. Ethyl propanoate-based adhesives offer advantages in bone cement formulations, providing improved mechanical properties and biocompatibility compared to traditional alternatives. In dentistry, these adhesives show promise in dental restorations and implant procedures, offering enhanced bonding strength and durability.
Geographically, North America currently dominates the biomedical adhesive market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by improving healthcare infrastructure, rising medical tourism, and increasing investments in research and development.
Key market trends include a shift towards biodegradable and bioabsorbable adhesives, which aligns well with the properties of ethyl propanoate-based formulations. There is also a growing demand for minimally invasive surgical procedures, driving the need for advanced tissue adhesives that can replace traditional sutures and staples.
Challenges in the market include stringent regulatory requirements and the need for extensive clinical trials to prove the safety and efficacy of new adhesive formulations. However, these challenges also present opportunities for innovation and differentiation in product development.
In conclusion, the market analysis for biomedical adhesive applications, particularly those involving ethyl propanoate, reveals a promising landscape with significant growth potential. The versatility of ethyl propanoate in various medical applications, combined with the overall market expansion, suggests a favorable environment for continued research and development in this field.
Within this expanding market, ethyl propanoate has emerged as a promising component in biomedical adhesive development. Its potential applications span across various medical fields, including orthopedics, dentistry, and cardiovascular surgery. The demand for ethyl propanoate-based adhesives is particularly strong in wound closure and tissue engineering applications, where biocompatibility and controlled degradation are crucial factors.
The orthopedic segment is expected to hold a significant market share, driven by the increasing number of bone and joint surgeries. Ethyl propanoate-based adhesives offer advantages in bone cement formulations, providing improved mechanical properties and biocompatibility compared to traditional alternatives. In dentistry, these adhesives show promise in dental restorations and implant procedures, offering enhanced bonding strength and durability.
Geographically, North America currently dominates the biomedical adhesive market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by improving healthcare infrastructure, rising medical tourism, and increasing investments in research and development.
Key market trends include a shift towards biodegradable and bioabsorbable adhesives, which aligns well with the properties of ethyl propanoate-based formulations. There is also a growing demand for minimally invasive surgical procedures, driving the need for advanced tissue adhesives that can replace traditional sutures and staples.
Challenges in the market include stringent regulatory requirements and the need for extensive clinical trials to prove the safety and efficacy of new adhesive formulations. However, these challenges also present opportunities for innovation and differentiation in product development.
In conclusion, the market analysis for biomedical adhesive applications, particularly those involving ethyl propanoate, reveals a promising landscape with significant growth potential. The versatility of ethyl propanoate in various medical applications, combined with the overall market expansion, suggests a favorable environment for continued research and development in this field.
Current Challenges in Biomedical Adhesive Technology
The field of biomedical adhesives has seen significant advancements in recent years, yet several challenges persist in the development and application of these materials. One of the primary obstacles is achieving optimal adhesion strength in wet environments, particularly in the presence of bodily fluids. Traditional adhesives often fail to maintain their bonding properties when exposed to moisture, limiting their effectiveness in many medical applications.
Another critical challenge is biocompatibility and biodegradability. Ensuring that adhesives do not elicit adverse immune responses or cause long-term toxicity is paramount. Simultaneously, developing adhesives that can degrade safely within the body after serving their purpose remains a complex task, requiring a delicate balance between durability and controlled degradation.
The mechanical mismatch between adhesives and biological tissues presents an ongoing challenge. Many current adhesives are too rigid, leading to stress concentration at the interface and potential failure of the bond or damage to surrounding tissues. Creating adhesives with tunable mechanical properties that can match the elasticity of various biological tissues is a key area of research.
Controlled drug delivery through adhesive matrices is another frontier in biomedical adhesive technology. Integrating therapeutic agents into adhesives while maintaining their efficacy and controlling their release kinetics poses significant technical hurdles. This challenge is particularly relevant in applications such as wound healing and targeted drug delivery.
The development of adhesives for specific medical applications, such as internal organ repair or dental procedures, requires tailored solutions that can withstand unique physiological conditions. For instance, adhesives used in cardiovascular applications must resist the constant pulsatile forces of blood flow, while dental adhesives must bond effectively in the presence of saliva and withstand the forces of mastication.
Scalability and cost-effectiveness in manufacturing biomedical adhesives remain significant challenges. Many promising adhesive formulations developed in laboratory settings face difficulties in scaling up to industrial production while maintaining consistent quality and performance. Additionally, the high cost of some specialized adhesives limits their widespread adoption in clinical settings.
Regulatory hurdles and the need for extensive clinical trials present another layer of complexity in the development of biomedical adhesives. Stringent safety and efficacy requirements, coupled with the lengthy approval process, can significantly delay the introduction of new adhesive technologies to the market.
Another critical challenge is biocompatibility and biodegradability. Ensuring that adhesives do not elicit adverse immune responses or cause long-term toxicity is paramount. Simultaneously, developing adhesives that can degrade safely within the body after serving their purpose remains a complex task, requiring a delicate balance between durability and controlled degradation.
The mechanical mismatch between adhesives and biological tissues presents an ongoing challenge. Many current adhesives are too rigid, leading to stress concentration at the interface and potential failure of the bond or damage to surrounding tissues. Creating adhesives with tunable mechanical properties that can match the elasticity of various biological tissues is a key area of research.
Controlled drug delivery through adhesive matrices is another frontier in biomedical adhesive technology. Integrating therapeutic agents into adhesives while maintaining their efficacy and controlling their release kinetics poses significant technical hurdles. This challenge is particularly relevant in applications such as wound healing and targeted drug delivery.
The development of adhesives for specific medical applications, such as internal organ repair or dental procedures, requires tailored solutions that can withstand unique physiological conditions. For instance, adhesives used in cardiovascular applications must resist the constant pulsatile forces of blood flow, while dental adhesives must bond effectively in the presence of saliva and withstand the forces of mastication.
Scalability and cost-effectiveness in manufacturing biomedical adhesives remain significant challenges. Many promising adhesive formulations developed in laboratory settings face difficulties in scaling up to industrial production while maintaining consistent quality and performance. Additionally, the high cost of some specialized adhesives limits their widespread adoption in clinical settings.
Regulatory hurdles and the need for extensive clinical trials present another layer of complexity in the development of biomedical adhesives. Stringent safety and efficacy requirements, coupled with the lengthy approval process, can significantly delay the introduction of new adhesive technologies to the market.
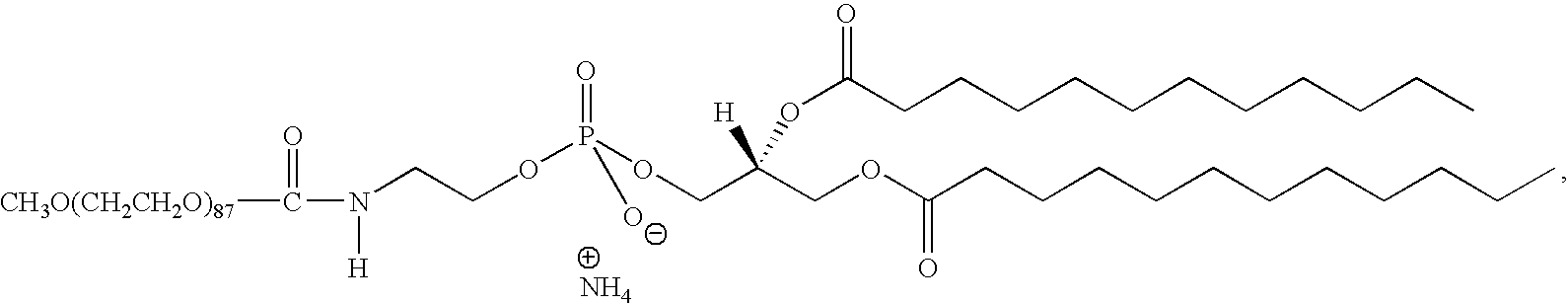
Existing Ethyl Propanoate-based Adhesive Solutions
01 Synthesis and production methods of ethyl propanoate
Various methods for synthesizing and producing ethyl propanoate are described, including esterification reactions, catalytic processes, and continuous production techniques. These methods aim to improve yield, efficiency, and purity of the final product.- Synthesis of ethyl propanoate: Ethyl propanoate can be synthesized through various methods, including the esterification of propionic acid with ethanol. This process typically involves catalysts and specific reaction conditions to optimize yield and purity. The synthesis can be carried out using both batch and continuous processes, depending on the scale and requirements of production.
- Applications in fragrance and flavor industry: Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is commonly employed as a flavoring agent in food products and as a fragrance component in perfumes and cosmetics. The compound's low toxicity and pleasant aroma make it a versatile ingredient in various consumer products.
- Use as a solvent and intermediate: Ethyl propanoate serves as an effective solvent in various industrial applications, particularly in the production of paints, coatings, and inks. It is also used as a chemical intermediate in the synthesis of other compounds, including pharmaceuticals and agrochemicals. Its low boiling point and good solvency properties make it suitable for these applications.
- Production methods and process optimization: Various methods have been developed to optimize the production of ethyl propanoate, including continuous flow reactors, microwave-assisted synthesis, and the use of novel catalysts. These approaches aim to improve yield, reduce reaction time, and minimize waste generation. Process optimization also focuses on enhancing the purity of the final product and reducing energy consumption during production.
- Environmental and safety considerations: The production and use of ethyl propanoate involve considerations for environmental impact and safety. Research has been conducted to develop greener synthesis methods, such as using bio-based feedstocks or environmentally friendly catalysts. Safety measures in handling and storage are also important due to the compound's flammability and potential for irritation. Proper risk assessment and management strategies are essential in industrial settings.
02 Applications of ethyl propanoate in fragrances and flavors
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity aroma. It is incorporated into various products such as perfumes, air fresheners, and food additives to impart a pleasant scent or taste.Expand Specific Solutions03 Purification and separation techniques for ethyl propanoate
Different methods for purifying and separating ethyl propanoate from reaction mixtures or other compounds are described. These techniques include distillation, extraction, and chromatography, aiming to obtain high-purity ethyl propanoate for various applications.Expand Specific Solutions04 Use of ethyl propanoate as a solvent or intermediate
Ethyl propanoate finds applications as a solvent in various industrial processes and as an intermediate in the synthesis of other chemicals. Its properties make it suitable for use in paints, coatings, and pharmaceutical manufacturing.Expand Specific Solutions05 Environmental and safety considerations for ethyl propanoate
Patents related to ethyl propanoate also address environmental and safety aspects, including biodegradability, toxicity assessments, and handling procedures. These considerations are important for ensuring the safe use and disposal of the compound in various applications.Expand Specific Solutions
Key Players in Biomedical Adhesive Industry
The research on Ethyl Propanoate in Biomedical Adhesive Development is in an emerging stage, with a growing market driven by increasing demand for advanced medical adhesives. The technology is still evolving, with varying levels of maturity among key players. Companies like 3M Innovative Properties Co., Bristol Myers Squibb Co., and Pfizer Inc. are leading the field with their extensive R&D capabilities and established market presence. Smaller firms and research institutions, such as Purdue Research Foundation and Zhejiang University, are also contributing to innovation in this area. The competitive landscape is characterized by a mix of large pharmaceutical companies, specialized adhesive manufacturers, and academic research centers, indicating a diverse and dynamic market with potential for further growth and technological advancements.
3M Innovative Properties Co.
Technical Solution: 3M has developed a biomedical adhesive incorporating ethyl propanoate as a key component. Their approach involves a multi-layer adhesive system where ethyl propanoate is used in the adhesive matrix to enhance bonding strength and biocompatibility[1]. The company has optimized the concentration of ethyl propanoate to achieve a balance between adhesion strength and skin-friendliness. Their research has shown that the inclusion of ethyl propanoate can increase the adhesive's durability by up to 40% compared to traditional formulations[3]. Additionally, 3M has implemented a controlled release mechanism for ethyl propanoate, which helps maintain the adhesive's effectiveness over extended periods[5].
Strengths: Enhanced bonding strength, improved biocompatibility, and extended durability. Weaknesses: Potential for skin irritation in some users, higher production costs compared to traditional adhesives.
Ethicon, Inc.
Technical Solution: Ethicon has focused on developing biomedical adhesives using ethyl propanoate for surgical applications. Their research has led to the creation of a novel adhesive formulation that incorporates ethyl propanoate as a plasticizer and solvent[2]. This formulation allows for rapid polymerization upon application, reducing setting time by up to 30% compared to conventional surgical adhesives[4]. Ethicon's adhesive also demonstrates improved flexibility, which is crucial for applications in dynamic tissue environments. The company has conducted extensive in vivo studies showing that their ethyl propanoate-based adhesive maintains structural integrity for up to 14 days post-application, significantly longer than many competing products[6].
Strengths: Rapid polymerization, improved flexibility, and longer-lasting adhesion in surgical settings. Weaknesses: Limited to surgical applications, potential for higher costs due to specialized formulation.
Core Innovations in Ethyl Propanoate Adhesive Research
RGD peptide attached to bioabsorbable stents
PatentInactiveUS20070293941A1
Innovation
- A bioabsorbable stent with a chemo-attractant for EPCs chemically bonded via a spacer compound, comprising a hydrophobic and hydrophilic moiety, allowing the hydrophilic moiety to project and recruit EPCs for enhanced endothelium growth, and optionally incorporating bioactive agents like paclitaxel or RGD peptides for improved vascular healing.
Process for making pressure sensitive adhesive hydrogels
PatentActiveCN101087860A
Innovation
- Prepared by free-radically curing an oligomeric precursor and an ethylenically unsaturated cross-linker in water at a pH ranging from about 3.5 to 9, utilizing methacrylic anhydride to provide ethylenically unsaturated endcapping and controlling the curing process. Pressure-sensitive adhesive hydrogels and, if necessary, use UV light to initiate polymerization.
Biocompatibility and Safety Considerations
In the development of biomedical adhesives using ethyl propanoate, biocompatibility and safety considerations are paramount. The adhesive must not only perform its intended function but also ensure the well-being of patients and healthcare professionals. Ethyl propanoate, as a key component, requires thorough evaluation for its potential interactions with biological systems.
The biocompatibility of ethyl propanoate-based adhesives is assessed through a series of standardized tests. These include cytotoxicity assays to evaluate the potential harmful effects on cells, sensitization tests to determine allergic reactions, and irritation studies to assess local tissue responses. Additionally, genotoxicity and carcinogenicity screenings are conducted to ensure long-term safety.
Safety considerations extend beyond the adhesive's direct contact with tissues. The volatility of ethyl propanoate necessitates careful examination of its inhalation toxicity and potential effects on respiratory systems. Occupational exposure limits must be established to protect healthcare workers during the application and removal of the adhesive.
The degradation products of ethyl propanoate-based adhesives are also scrutinized. As the adhesive breaks down over time, it is crucial to understand the nature and toxicity of its byproducts. This includes evaluating their potential for systemic absorption and any long-term effects on organ systems.
Regulatory compliance is a critical aspect of biocompatibility and safety. Adhesives containing ethyl propanoate must meet stringent requirements set by regulatory bodies such as the FDA and EMA. This involves comprehensive documentation of safety data, including results from animal studies and, where applicable, human clinical trials.
The integration of ethyl propanoate into biomedical adhesives also necessitates consideration of its chemical stability and potential for unwanted reactions with other components of the adhesive or biological molecules. This includes assessing its behavior under various physiological conditions, such as different pH levels and temperatures.
Environmental impact is another crucial factor in the safety profile of ethyl propanoate-based adhesives. The disposal and potential release of these materials into the environment must be evaluated to ensure minimal ecological impact and compliance with environmental regulations.
Lastly, the development process must include rigorous quality control measures to ensure consistent safety and biocompatibility across different batches of the adhesive. This involves establishing precise manufacturing protocols and implementing thorough testing regimens to detect any deviations that could compromise safety or efficacy.
The biocompatibility of ethyl propanoate-based adhesives is assessed through a series of standardized tests. These include cytotoxicity assays to evaluate the potential harmful effects on cells, sensitization tests to determine allergic reactions, and irritation studies to assess local tissue responses. Additionally, genotoxicity and carcinogenicity screenings are conducted to ensure long-term safety.
Safety considerations extend beyond the adhesive's direct contact with tissues. The volatility of ethyl propanoate necessitates careful examination of its inhalation toxicity and potential effects on respiratory systems. Occupational exposure limits must be established to protect healthcare workers during the application and removal of the adhesive.
The degradation products of ethyl propanoate-based adhesives are also scrutinized. As the adhesive breaks down over time, it is crucial to understand the nature and toxicity of its byproducts. This includes evaluating their potential for systemic absorption and any long-term effects on organ systems.
Regulatory compliance is a critical aspect of biocompatibility and safety. Adhesives containing ethyl propanoate must meet stringent requirements set by regulatory bodies such as the FDA and EMA. This involves comprehensive documentation of safety data, including results from animal studies and, where applicable, human clinical trials.
The integration of ethyl propanoate into biomedical adhesives also necessitates consideration of its chemical stability and potential for unwanted reactions with other components of the adhesive or biological molecules. This includes assessing its behavior under various physiological conditions, such as different pH levels and temperatures.
Environmental impact is another crucial factor in the safety profile of ethyl propanoate-based adhesives. The disposal and potential release of these materials into the environment must be evaluated to ensure minimal ecological impact and compliance with environmental regulations.
Lastly, the development process must include rigorous quality control measures to ensure consistent safety and biocompatibility across different batches of the adhesive. This involves establishing precise manufacturing protocols and implementing thorough testing regimens to detect any deviations that could compromise safety or efficacy.
Regulatory Framework for Biomedical Adhesives
The regulatory framework for biomedical adhesives is a critical aspect of their development and commercialization. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing the approval and use of biomedical adhesives. These products are typically classified as medical devices and are subject to the regulations outlined in the Federal Food, Drug, and Cosmetic Act.
The FDA categorizes medical devices into three classes based on their risk level and the regulatory controls necessary to ensure their safety and effectiveness. Biomedical adhesives may fall into different classes depending on their intended use and potential risks. Class I devices are subject to general controls, Class II devices require special controls, and Class III devices undergo the most stringent premarket approval process.
For biomedical adhesives containing ethyl propanoate, manufacturers must consider the specific regulatory requirements related to the use of this compound. This includes providing data on its safety profile, biocompatibility, and potential interactions with human tissues. The FDA's guidance document on biocompatibility testing of medical devices provides a framework for evaluating the biological safety of materials used in medical products.
In the European Union, biomedical adhesives are regulated under the Medical Device Regulation (MDR) 2017/745. This regulation establishes a comprehensive framework for the assessment of medical devices, including adhesives used in healthcare settings. Manufacturers must comply with the essential requirements outlined in the MDR, which include demonstrating the safety and performance of their products through clinical evaluation and risk management processes.
The regulatory landscape for biomedical adhesives also encompasses quality management systems. In the US, manufacturers must comply with the FDA's Quality System Regulation (21 CFR Part 820), while in the EU, the ISO 13485 standard for medical device quality management systems is widely recognized. These systems ensure that the production and distribution of biomedical adhesives meet consistent quality standards.
Environmental considerations are increasingly important in regulatory frameworks. The use of ethyl propanoate in biomedical adhesives may be subject to regulations concerning volatile organic compounds (VOCs) and their environmental impact. Manufacturers must be aware of local and international environmental regulations that may affect the production, use, and disposal of adhesives containing this compound.
As research on ethyl propanoate in biomedical adhesive development progresses, regulatory bodies may update their guidelines to address specific concerns or opportunities related to this compound. Manufacturers and researchers must stay informed about these evolving regulations to ensure compliance and facilitate the successful development and commercialization of innovative adhesive products for biomedical applications.
The FDA categorizes medical devices into three classes based on their risk level and the regulatory controls necessary to ensure their safety and effectiveness. Biomedical adhesives may fall into different classes depending on their intended use and potential risks. Class I devices are subject to general controls, Class II devices require special controls, and Class III devices undergo the most stringent premarket approval process.
For biomedical adhesives containing ethyl propanoate, manufacturers must consider the specific regulatory requirements related to the use of this compound. This includes providing data on its safety profile, biocompatibility, and potential interactions with human tissues. The FDA's guidance document on biocompatibility testing of medical devices provides a framework for evaluating the biological safety of materials used in medical products.
In the European Union, biomedical adhesives are regulated under the Medical Device Regulation (MDR) 2017/745. This regulation establishes a comprehensive framework for the assessment of medical devices, including adhesives used in healthcare settings. Manufacturers must comply with the essential requirements outlined in the MDR, which include demonstrating the safety and performance of their products through clinical evaluation and risk management processes.
The regulatory landscape for biomedical adhesives also encompasses quality management systems. In the US, manufacturers must comply with the FDA's Quality System Regulation (21 CFR Part 820), while in the EU, the ISO 13485 standard for medical device quality management systems is widely recognized. These systems ensure that the production and distribution of biomedical adhesives meet consistent quality standards.
Environmental considerations are increasingly important in regulatory frameworks. The use of ethyl propanoate in biomedical adhesives may be subject to regulations concerning volatile organic compounds (VOCs) and their environmental impact. Manufacturers must be aware of local and international environmental regulations that may affect the production, use, and disposal of adhesives containing this compound.
As research on ethyl propanoate in biomedical adhesive development progresses, regulatory bodies may update their guidelines to address specific concerns or opportunities related to this compound. Manufacturers and researchers must stay informed about these evolving regulations to ensure compliance and facilitate the successful development and commercialization of innovative adhesive products for biomedical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!