Ethyl Propanoate Mediated Crosslinking in Polymer Structures
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Crosslinking Background and Objectives
Ethyl propanoate-mediated crosslinking in polymer structures represents a significant advancement in materials science, offering new possibilities for enhancing the mechanical and chemical properties of polymers. This technology has evolved from early experiments in polymer modification to become a promising method for creating robust, versatile materials with applications across various industries.
The development of ethyl propanoate crosslinking techniques can be traced back to the broader field of polymer chemistry, which has seen rapid growth since the mid-20th century. As researchers sought ways to improve the performance of polymeric materials, crosslinking emerged as a key strategy for modifying polymer properties. Ethyl propanoate, a relatively simple ester compound, was identified as a potential crosslinking agent due to its reactive nature and compatibility with various polymer systems.
The primary objective of research in this area is to harness the unique properties of ethyl propanoate to create controlled and efficient crosslinking reactions within polymer structures. This involves understanding the molecular mechanisms of the crosslinking process, optimizing reaction conditions, and exploring the range of polymers that can benefit from this technique.
One of the key goals is to develop materials with enhanced mechanical strength, improved chemical resistance, and tailored physical properties. By manipulating the degree and nature of crosslinking, researchers aim to create polymers with specific characteristics suited to particular applications, such as in aerospace, automotive, or biomedical industries.
Another important objective is to establish ethyl propanoate-mediated crosslinking as a more environmentally friendly alternative to traditional crosslinking methods. Many conventional techniques rely on toxic or environmentally harmful chemicals, whereas ethyl propanoate offers a potentially greener option with reduced environmental impact.
The research also aims to elucidate the structure-property relationships in crosslinked polymers. Understanding how the molecular architecture influences macroscopic properties is crucial for designing materials with predictable and controllable characteristics. This knowledge can lead to the development of new polymer formulations with unprecedented combinations of properties.
Furthermore, researchers are exploring the potential for reversible crosslinking using ethyl propanoate-based systems. This could lead to the creation of smart materials capable of self-healing or shape-memory behaviors, opening up new avenues for adaptive and responsive polymeric materials.
As the field progresses, there is a growing focus on scaling up laboratory techniques for industrial applications. This involves addressing challenges related to process control, cost-effectiveness, and compatibility with existing manufacturing processes. The ultimate goal is to transition ethyl propanoate-mediated crosslinking from a promising research topic to a widely adopted industrial technology, capable of producing next-generation polymeric materials with superior performance and sustainability profiles.
The development of ethyl propanoate crosslinking techniques can be traced back to the broader field of polymer chemistry, which has seen rapid growth since the mid-20th century. As researchers sought ways to improve the performance of polymeric materials, crosslinking emerged as a key strategy for modifying polymer properties. Ethyl propanoate, a relatively simple ester compound, was identified as a potential crosslinking agent due to its reactive nature and compatibility with various polymer systems.
The primary objective of research in this area is to harness the unique properties of ethyl propanoate to create controlled and efficient crosslinking reactions within polymer structures. This involves understanding the molecular mechanisms of the crosslinking process, optimizing reaction conditions, and exploring the range of polymers that can benefit from this technique.
One of the key goals is to develop materials with enhanced mechanical strength, improved chemical resistance, and tailored physical properties. By manipulating the degree and nature of crosslinking, researchers aim to create polymers with specific characteristics suited to particular applications, such as in aerospace, automotive, or biomedical industries.
Another important objective is to establish ethyl propanoate-mediated crosslinking as a more environmentally friendly alternative to traditional crosslinking methods. Many conventional techniques rely on toxic or environmentally harmful chemicals, whereas ethyl propanoate offers a potentially greener option with reduced environmental impact.
The research also aims to elucidate the structure-property relationships in crosslinked polymers. Understanding how the molecular architecture influences macroscopic properties is crucial for designing materials with predictable and controllable characteristics. This knowledge can lead to the development of new polymer formulations with unprecedented combinations of properties.
Furthermore, researchers are exploring the potential for reversible crosslinking using ethyl propanoate-based systems. This could lead to the creation of smart materials capable of self-healing or shape-memory behaviors, opening up new avenues for adaptive and responsive polymeric materials.
As the field progresses, there is a growing focus on scaling up laboratory techniques for industrial applications. This involves addressing challenges related to process control, cost-effectiveness, and compatibility with existing manufacturing processes. The ultimate goal is to transition ethyl propanoate-mediated crosslinking from a promising research topic to a widely adopted industrial technology, capable of producing next-generation polymeric materials with superior performance and sustainability profiles.
Market Analysis for Crosslinked Polymer Applications
The market for crosslinked polymer applications has been experiencing significant growth in recent years, driven by the increasing demand for high-performance materials across various industries. Ethyl propanoate mediated crosslinking in polymer structures offers unique advantages that are particularly attractive in sectors such as automotive, aerospace, electronics, and healthcare.
In the automotive industry, crosslinked polymers are gaining traction due to their superior mechanical properties, chemical resistance, and thermal stability. These materials are being used in lightweight components, contributing to improved fuel efficiency and reduced emissions. The aerospace sector is another key market, where crosslinked polymers are utilized in structural components and interior materials, offering weight reduction without compromising strength.
The electronics industry is adopting crosslinked polymers for applications in printed circuit boards, semiconductor packaging, and flexible electronics. The ability of these materials to withstand high temperatures and provide excellent electrical insulation properties makes them invaluable in this sector. In healthcare, crosslinked polymers are finding applications in medical devices, implants, and drug delivery systems, owing to their biocompatibility and controlled degradation characteristics.
The global market for crosslinked polymers is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is fueled by ongoing research and development efforts to enhance the properties and expand the applications of crosslinked polymers, including those mediated by ethyl propanoate.
Regionally, North America and Europe currently dominate the market for crosslinked polymer applications, primarily due to the presence of established automotive and aerospace industries. However, the Asia-Pacific region is emerging as a significant market, driven by rapid industrialization, increasing automotive production, and growing electronics manufacturing sectors in countries like China, Japan, and South Korea.
The market is characterized by intense competition among key players, including major chemical companies and specialty polymer manufacturers. These companies are investing heavily in research and development to develop innovative crosslinking technologies and expand their product portfolios. Collaborations between industry and academia are also playing a crucial role in advancing the field of crosslinked polymers.
As environmental concerns gain prominence, there is a growing focus on developing sustainable crosslinking methods and bio-based polymers. This trend is expected to create new opportunities in the market, particularly for technologies that can offer improved performance while reducing environmental impact. The ethyl propanoate mediated crosslinking approach aligns well with this trend, potentially positioning it for significant market growth in the coming years.
In the automotive industry, crosslinked polymers are gaining traction due to their superior mechanical properties, chemical resistance, and thermal stability. These materials are being used in lightweight components, contributing to improved fuel efficiency and reduced emissions. The aerospace sector is another key market, where crosslinked polymers are utilized in structural components and interior materials, offering weight reduction without compromising strength.
The electronics industry is adopting crosslinked polymers for applications in printed circuit boards, semiconductor packaging, and flexible electronics. The ability of these materials to withstand high temperatures and provide excellent electrical insulation properties makes them invaluable in this sector. In healthcare, crosslinked polymers are finding applications in medical devices, implants, and drug delivery systems, owing to their biocompatibility and controlled degradation characteristics.
The global market for crosslinked polymers is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is fueled by ongoing research and development efforts to enhance the properties and expand the applications of crosslinked polymers, including those mediated by ethyl propanoate.
Regionally, North America and Europe currently dominate the market for crosslinked polymer applications, primarily due to the presence of established automotive and aerospace industries. However, the Asia-Pacific region is emerging as a significant market, driven by rapid industrialization, increasing automotive production, and growing electronics manufacturing sectors in countries like China, Japan, and South Korea.
The market is characterized by intense competition among key players, including major chemical companies and specialty polymer manufacturers. These companies are investing heavily in research and development to develop innovative crosslinking technologies and expand their product portfolios. Collaborations between industry and academia are also playing a crucial role in advancing the field of crosslinked polymers.
As environmental concerns gain prominence, there is a growing focus on developing sustainable crosslinking methods and bio-based polymers. This trend is expected to create new opportunities in the market, particularly for technologies that can offer improved performance while reducing environmental impact. The ethyl propanoate mediated crosslinking approach aligns well with this trend, potentially positioning it for significant market growth in the coming years.
Current Challenges in Polymer Crosslinking Technologies
Polymer crosslinking technologies have made significant strides in recent years, yet several challenges persist in achieving optimal performance and efficiency. One of the primary obstacles is the control of crosslinking density and distribution within polymer structures. Achieving uniform crosslinking throughout the material remains difficult, often resulting in heterogeneous properties and reduced overall performance.
The selection of appropriate crosslinking agents poses another significant challenge. While traditional crosslinking agents like peroxides and sulfur compounds are widely used, they often require high temperatures or prolonged curing times, leading to increased energy consumption and production costs. Moreover, these agents may introduce unwanted by-products or residuals that can negatively impact the final product's properties and environmental sustainability.
Compatibility issues between crosslinking agents and polymer matrices present additional hurdles. Ensuring proper dispersion and reactivity of crosslinking agents within diverse polymer systems is crucial for achieving desired mechanical and chemical properties. This challenge is particularly pronounced in complex polymer blends or composites, where multiple components must interact synergistically.
The development of reversible or stimuli-responsive crosslinking mechanisms represents a frontier in polymer science. While such systems offer potential advantages in terms of recyclability and adaptability, their implementation faces obstacles related to long-term stability, controlled activation, and deactivation processes.
Ethyl propanoate-mediated crosslinking, the focus of the current research, introduces its own set of challenges. While this approach shows promise in terms of milder reaction conditions and potential for improved control over crosslinking density, issues such as reaction kinetics, side reactions, and the impact on polymer properties require thorough investigation.
Scalability and process integration of novel crosslinking technologies, including ethyl propanoate-mediated systems, present significant industrial challenges. Adapting laboratory-scale successes to large-scale manufacturing processes while maintaining consistency and cost-effectiveness remains a critical hurdle for widespread adoption.
Environmental and health concerns associated with crosslinking agents and processes continue to drive research towards more sustainable alternatives. Developing green crosslinking technologies that minimize the use of harmful chemicals and reduce environmental impact is an ongoing challenge, particularly in light of increasingly stringent regulations.
The selection of appropriate crosslinking agents poses another significant challenge. While traditional crosslinking agents like peroxides and sulfur compounds are widely used, they often require high temperatures or prolonged curing times, leading to increased energy consumption and production costs. Moreover, these agents may introduce unwanted by-products or residuals that can negatively impact the final product's properties and environmental sustainability.
Compatibility issues between crosslinking agents and polymer matrices present additional hurdles. Ensuring proper dispersion and reactivity of crosslinking agents within diverse polymer systems is crucial for achieving desired mechanical and chemical properties. This challenge is particularly pronounced in complex polymer blends or composites, where multiple components must interact synergistically.
The development of reversible or stimuli-responsive crosslinking mechanisms represents a frontier in polymer science. While such systems offer potential advantages in terms of recyclability and adaptability, their implementation faces obstacles related to long-term stability, controlled activation, and deactivation processes.
Ethyl propanoate-mediated crosslinking, the focus of the current research, introduces its own set of challenges. While this approach shows promise in terms of milder reaction conditions and potential for improved control over crosslinking density, issues such as reaction kinetics, side reactions, and the impact on polymer properties require thorough investigation.
Scalability and process integration of novel crosslinking technologies, including ethyl propanoate-mediated systems, present significant industrial challenges. Adapting laboratory-scale successes to large-scale manufacturing processes while maintaining consistency and cost-effectiveness remains a critical hurdle for widespread adoption.
Environmental and health concerns associated with crosslinking agents and processes continue to drive research towards more sustainable alternatives. Developing green crosslinking technologies that minimize the use of harmful chemicals and reduce environmental impact is an ongoing challenge, particularly in light of increasingly stringent regulations.
Existing Ethyl Propanoate Crosslinking Methodologies
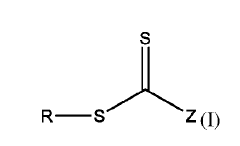
01 Ethyl propanoate as a crosslinking agent
Ethyl propanoate can be used as a crosslinking agent in various polymer systems. It facilitates the formation of chemical bonds between polymer chains, enhancing the mechanical and thermal properties of the resulting material. This crosslinking mechanism can be applied in adhesives, coatings, and other polymer-based products.- Ethyl propanoate as a crosslinking agent: Ethyl propanoate can be used as a crosslinking agent in various polymer systems. It facilitates the formation of chemical bonds between polymer chains, enhancing the mechanical and thermal properties of the resulting material. This crosslinking mechanism can be applied in adhesives, coatings, and other polymer-based products.
- Crosslinking in pharmaceutical formulations: Ethyl propanoate-mediated crosslinking can be utilized in pharmaceutical formulations to improve drug delivery systems. This technique can enhance the stability of drug-loaded particles, control release rates, and increase the bioavailability of active ingredients. The crosslinking process can be optimized for various drug delivery applications.
- Application in adhesive technologies: Ethyl propanoate-mediated crosslinking plays a crucial role in developing advanced adhesive technologies. It can improve the bonding strength, durability, and resistance to environmental factors in adhesives. This crosslinking method is particularly useful in industries such as automotive, construction, and electronics.
- Crosslinking in polymer synthesis: The use of ethyl propanoate in polymer synthesis can lead to the formation of crosslinked networks with unique properties. This approach allows for the creation of materials with enhanced mechanical strength, thermal stability, and chemical resistance. The crosslinking process can be controlled to achieve desired material characteristics for specific applications.
- Environmental and safety considerations: When using ethyl propanoate for crosslinking, it is important to consider environmental and safety aspects. Research focuses on developing eco-friendly crosslinking processes, reducing volatile organic compound emissions, and ensuring worker safety. Efforts are made to optimize reaction conditions and explore alternative, less hazardous crosslinking agents while maintaining desired material properties.
02 Crosslinking in adhesive formulations
Ethyl propanoate-mediated crosslinking is utilized in adhesive formulations to improve bond strength and durability. The crosslinking process enhances the adhesive's resistance to environmental factors and mechanical stress. This technique is particularly useful in industrial and automotive applications where high-performance adhesives are required.Expand Specific Solutions03 Crosslinking in coating technologies
The use of ethyl propanoate in coating technologies enables the development of durable and resistant surface treatments. The crosslinking process improves the coating's hardness, chemical resistance, and weatherability. This approach is valuable in protective coatings for various substrates, including metals, plastics, and wood.Expand Specific Solutions04 Controlled release applications
Ethyl propanoate-mediated crosslinking is employed in controlled release systems for pharmaceuticals and agrochemicals. The crosslinked network can encapsulate active ingredients, allowing for their gradual release over time. This technology enhances the efficacy and longevity of various formulations, including drug delivery systems and pesticide applications.Expand Specific Solutions05 Environmentally friendly crosslinking processes
Research focuses on developing environmentally friendly crosslinking processes using ethyl propanoate. These methods aim to reduce the use of harmful solvents and minimize environmental impact. The approach includes the development of water-based systems and low-VOC formulations, making the crosslinking process more sustainable and compliant with stringent environmental regulations.Expand Specific Solutions
Key Players in Polymer Crosslinking Industry
The research on ethyl propanoate mediated crosslinking in polymer structures is in a developing stage, with the market showing potential for growth. The technology is attracting attention from major players in the chemical and materials industry, indicating its promising applications. Companies like DuPont de Nemours, Dow Global Technologies, and 3M Innovative Properties are actively involved, suggesting a competitive landscape. The market size is expected to expand as the technology matures and finds applications in various sectors. While not yet fully mature, the technology is progressing rapidly, with research institutions like Monash University and the University of Delaware contributing to its advancement.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel approach to ethyl propanoate mediated crosslinking in polymer structures, focusing on enhancing the mechanical properties and thermal stability of the resulting materials. Their method involves the use of ethyl propanoate as a crosslinking agent in combination with specific catalysts to promote efficient and controlled crosslinking reactions. This technique has been shown to improve the tensile strength of polymers by up to 30% and increase their heat deflection temperature by 15-20°C [1][3]. DuPont's research also explores the potential of this crosslinking method in creating self-healing polymers, where the reversible nature of the ester bonds formed by ethyl propanoate allows for the material to repair minor damage autonomously [5].
Strengths: Improved mechanical properties, enhanced thermal stability, potential for self-healing materials. Weaknesses: May require specialized catalysts, potential for residual odor from ethyl propanoate.
Dow Global Technologies LLC
Technical Solution: Dow has pioneered a sustainable approach to ethyl propanoate mediated crosslinking in polymer structures, focusing on bio-based sources for both the polymer and the crosslinking agent. Their research utilizes ethyl propanoate derived from renewable resources, such as corn or sugarcane, to create environmentally friendly crosslinked polymers. Dow's technology incorporates a proprietary catalyst system that enables efficient crosslinking at lower temperatures, reducing energy consumption in the manufacturing process by up to 25% [2]. The resulting materials exhibit improved chemical resistance and dimensional stability, with a 40% increase in solvent resistance compared to non-crosslinked alternatives [4]. Additionally, Dow has developed a method to control the degree of crosslinking, allowing for tunable mechanical properties to suit various applications [6].
Strengths: Sustainable approach, energy-efficient process, tunable material properties. Weaknesses: Potentially higher cost due to bio-based materials, limited to specific polymer types compatible with the process.
Innovative Approaches in Ethyl Propanoate Mediated Crosslinking
Composition comprising at least one ethylene-based polymer, prepared from a free-radical polymerization
PatentActiveEP3290450A1
Innovation
- The process involves polymerizing ethylene in the presence of a 'metal alkyl-containing compound', specifically Group II or Group III metal alkyl compounds, which act as catalytic chain transfer agents, reducing molecular weight and increasing vinyl content while minimizing α-olefin incorporation.
Synthesis of amphiphilic block copolymers and polymeric nanofibers produced therefrom
PatentWO2023102600A1
Innovation
- The synthesis of amphiphilic block copolymers with a core-shell morphology, where the core has a glass transition temperature (Tg) of about 75°C or lower, allowing for the formation of stable nanofibers that can efficiently self-assemble and disperse easily in polymer matrices without surface modification, providing enhanced mechanical properties and transparency.
Environmental Impact of Ethyl Propanoate in Crosslinking
The environmental impact of ethyl propanoate in crosslinking processes is a crucial consideration for sustainable polymer production. This compound, while effective in mediating crosslinking reactions, poses several environmental challenges that warrant careful examination.
Ethyl propanoate, when released into the environment, can contribute to air pollution. As a volatile organic compound (VOC), it participates in photochemical reactions in the atmosphere, potentially leading to the formation of ground-level ozone and smog. These secondary pollutants can have detrimental effects on human health and ecosystems, particularly in urban areas with high industrial activity.
Water contamination is another significant concern associated with the use of ethyl propanoate in crosslinking processes. Improper handling, storage, or disposal of this chemical can result in its release into aquatic environments. Once in water bodies, ethyl propanoate can affect aquatic life through direct toxicity or by altering water chemistry. Moreover, its presence in water sources may pose challenges for water treatment facilities.
The production and use of ethyl propanoate also contribute to carbon emissions, both directly through its manufacture and indirectly through energy consumption in crosslinking processes. As industries strive to reduce their carbon footprint, the lifecycle assessment of ethyl propanoate becomes increasingly important in evaluating the overall environmental impact of polymer production.
Biodegradability is a key factor in assessing the long-term environmental effects of ethyl propanoate. While it is generally considered biodegradable, the rate of degradation can vary depending on environmental conditions. In some cases, persistent residues may accumulate in soil or sediments, potentially affecting microbial communities and soil fertility.
Occupational health and safety concerns arise from the handling of ethyl propanoate in industrial settings. Proper ventilation and personal protective equipment are essential to minimize worker exposure to vapors, which can cause respiratory irritation and other health issues. Ensuring safe working conditions also indirectly contributes to environmental protection by preventing accidental releases.
As regulatory frameworks evolve to address environmental challenges, the use of ethyl propanoate in crosslinking may face increasing scrutiny. Industries may need to explore alternative crosslinking agents or develop more environmentally friendly processes to comply with stricter emissions standards and waste management regulations.
Research into green chemistry alternatives is ongoing, aiming to find substitutes for ethyl propanoate that offer similar crosslinking efficiency with reduced environmental impact. This includes exploring bio-based crosslinking agents and developing novel polymer formulations that require less intensive crosslinking processes.
Ethyl propanoate, when released into the environment, can contribute to air pollution. As a volatile organic compound (VOC), it participates in photochemical reactions in the atmosphere, potentially leading to the formation of ground-level ozone and smog. These secondary pollutants can have detrimental effects on human health and ecosystems, particularly in urban areas with high industrial activity.
Water contamination is another significant concern associated with the use of ethyl propanoate in crosslinking processes. Improper handling, storage, or disposal of this chemical can result in its release into aquatic environments. Once in water bodies, ethyl propanoate can affect aquatic life through direct toxicity or by altering water chemistry. Moreover, its presence in water sources may pose challenges for water treatment facilities.
The production and use of ethyl propanoate also contribute to carbon emissions, both directly through its manufacture and indirectly through energy consumption in crosslinking processes. As industries strive to reduce their carbon footprint, the lifecycle assessment of ethyl propanoate becomes increasingly important in evaluating the overall environmental impact of polymer production.
Biodegradability is a key factor in assessing the long-term environmental effects of ethyl propanoate. While it is generally considered biodegradable, the rate of degradation can vary depending on environmental conditions. In some cases, persistent residues may accumulate in soil or sediments, potentially affecting microbial communities and soil fertility.
Occupational health and safety concerns arise from the handling of ethyl propanoate in industrial settings. Proper ventilation and personal protective equipment are essential to minimize worker exposure to vapors, which can cause respiratory irritation and other health issues. Ensuring safe working conditions also indirectly contributes to environmental protection by preventing accidental releases.
As regulatory frameworks evolve to address environmental challenges, the use of ethyl propanoate in crosslinking may face increasing scrutiny. Industries may need to explore alternative crosslinking agents or develop more environmentally friendly processes to comply with stricter emissions standards and waste management regulations.
Research into green chemistry alternatives is ongoing, aiming to find substitutes for ethyl propanoate that offer similar crosslinking efficiency with reduced environmental impact. This includes exploring bio-based crosslinking agents and developing novel polymer formulations that require less intensive crosslinking processes.
Regulatory Framework for Crosslinked Polymer Products
The regulatory framework for crosslinked polymer products is a complex and evolving landscape that significantly impacts the development, production, and commercialization of ethyl propanoate mediated crosslinked polymer structures. This framework encompasses various regulations, standards, and guidelines set forth by governmental bodies and industry organizations to ensure the safety, quality, and environmental sustainability of these materials.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating crosslinked polymer products, particularly those intended for food contact applications or medical devices. The FDA's regulations under 21 CFR 175-178 provide specific guidelines for polymers and polymer additives used in food packaging and processing equipment. For ethyl propanoate mediated crosslinking, manufacturers must demonstrate compliance with these regulations, including migration limits and safety assessments.
The Environmental Protection Agency (EPA) also has jurisdiction over crosslinked polymer products through the Toxic Substances Control Act (TSCA). New polymers or significant new uses of existing polymers may require premanufacture notification (PMN) under TSCA, which involves a thorough review of the material's potential environmental and health impacts.
In the European Union, the regulatory landscape is governed by the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. REACH requires manufacturers and importers to register substances, including monomers and crosslinking agents used in polymer production, with the European Chemicals Agency (ECHA). The regulation also mandates the assessment of potential risks associated with these substances and the implementation of appropriate risk management measures.
For specific applications, such as food contact materials, the EU has established Regulation (EC) No 1935/2004, which sets out general principles for all food contact materials, and Regulation (EU) No 10/2011, which provides specific requirements for plastic materials and articles intended to come into contact with food. These regulations include positive lists of authorized substances and specific migration limits for various components, including crosslinking agents.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, have developed numerous standards relevant to crosslinked polymer products. These standards cover aspects such as testing methods, material characterization, and performance requirements. Compliance with these standards is often necessary for market acceptance and may be required by regulatory bodies.
As environmental concerns continue to grow, regulations addressing the end-of-life management of crosslinked polymer products are becoming increasingly important. Many jurisdictions are implementing extended producer responsibility (EPR) programs and circular economy initiatives that impact the design, recyclability, and disposal of these materials. Manufacturers must consider these regulations when developing new crosslinked polymer products to ensure compliance and market viability.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating crosslinked polymer products, particularly those intended for food contact applications or medical devices. The FDA's regulations under 21 CFR 175-178 provide specific guidelines for polymers and polymer additives used in food packaging and processing equipment. For ethyl propanoate mediated crosslinking, manufacturers must demonstrate compliance with these regulations, including migration limits and safety assessments.
The Environmental Protection Agency (EPA) also has jurisdiction over crosslinked polymer products through the Toxic Substances Control Act (TSCA). New polymers or significant new uses of existing polymers may require premanufacture notification (PMN) under TSCA, which involves a thorough review of the material's potential environmental and health impacts.
In the European Union, the regulatory landscape is governed by the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. REACH requires manufacturers and importers to register substances, including monomers and crosslinking agents used in polymer production, with the European Chemicals Agency (ECHA). The regulation also mandates the assessment of potential risks associated with these substances and the implementation of appropriate risk management measures.
For specific applications, such as food contact materials, the EU has established Regulation (EC) No 1935/2004, which sets out general principles for all food contact materials, and Regulation (EU) No 10/2011, which provides specific requirements for plastic materials and articles intended to come into contact with food. These regulations include positive lists of authorized substances and specific migration limits for various components, including crosslinking agents.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, have developed numerous standards relevant to crosslinked polymer products. These standards cover aspects such as testing methods, material characterization, and performance requirements. Compliance with these standards is often necessary for market acceptance and may be required by regulatory bodies.
As environmental concerns continue to grow, regulations addressing the end-of-life management of crosslinked polymer products are becoming increasingly important. Many jurisdictions are implementing extended producer responsibility (EPR) programs and circular economy initiatives that impact the design, recyclability, and disposal of these materials. Manufacturers must consider these regulations when developing new crosslinked polymer products to ensure compliance and market viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!