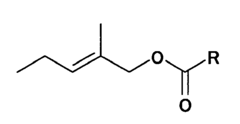
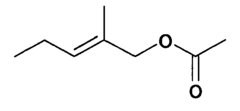
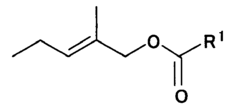
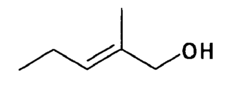
Evaluating Ethyl Propanoate Modified Tissue Engineering Scaffolds
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Scaffolds: Background and Objectives
Tissue engineering has emerged as a promising field in regenerative medicine, aiming to create functional substitutes for damaged tissues and organs. The development of suitable scaffolds plays a crucial role in this process, providing a structural framework for cell growth and tissue formation. In recent years, the modification of scaffolds with bioactive compounds has gained significant attention, with ethyl propanoate emerging as a potential candidate for enhancing scaffold properties.
Ethyl propanoate, also known as ethyl propionate, is an organic compound with the molecular formula C5H10O2. It is a colorless liquid with a fruity odor, commonly used in the food and fragrance industries. However, its potential applications in tissue engineering have only recently begun to be explored. The incorporation of ethyl propanoate into tissue engineering scaffolds aims to improve their biocompatibility, mechanical properties, and overall performance in supporting cell growth and tissue regeneration.
The primary objective of this technical research report is to evaluate the potential of ethyl propanoate-modified tissue engineering scaffolds. This evaluation encompasses a comprehensive analysis of the current state of the technology, its developmental trajectory, and the challenges that need to be addressed for successful implementation. By examining the background and setting clear objectives, we aim to provide a solid foundation for understanding the significance of this innovative approach in tissue engineering.
One of the key goals of this research is to assess the impact of ethyl propanoate modification on scaffold properties. This includes investigating how the compound affects the scaffold's porosity, mechanical strength, degradation rate, and ability to support cell adhesion and proliferation. Additionally, we aim to explore the potential synergistic effects between ethyl propanoate and other bioactive molecules commonly used in tissue engineering applications.
Another important objective is to identify the most suitable fabrication methods for incorporating ethyl propanoate into various scaffold materials. This involves evaluating different techniques such as electrospinning, 3D printing, and solvent casting, to determine which approaches yield the most effective and reproducible results. Furthermore, we seek to understand the optimal concentration and distribution of ethyl propanoate within the scaffold structure to maximize its beneficial effects.
As we delve into this emerging field, it is crucial to consider the broader implications of ethyl propanoate-modified scaffolds in the context of tissue engineering and regenerative medicine. This includes assessing their potential applications in specific tissue types, such as bone, cartilage, or soft tissues, and exploring their compatibility with various cell types and growth factors. By setting these comprehensive objectives, we aim to provide a thorough evaluation of the technology's potential and guide future research and development efforts in this promising area of tissue engineering.
Ethyl propanoate, also known as ethyl propionate, is an organic compound with the molecular formula C5H10O2. It is a colorless liquid with a fruity odor, commonly used in the food and fragrance industries. However, its potential applications in tissue engineering have only recently begun to be explored. The incorporation of ethyl propanoate into tissue engineering scaffolds aims to improve their biocompatibility, mechanical properties, and overall performance in supporting cell growth and tissue regeneration.
The primary objective of this technical research report is to evaluate the potential of ethyl propanoate-modified tissue engineering scaffolds. This evaluation encompasses a comprehensive analysis of the current state of the technology, its developmental trajectory, and the challenges that need to be addressed for successful implementation. By examining the background and setting clear objectives, we aim to provide a solid foundation for understanding the significance of this innovative approach in tissue engineering.
One of the key goals of this research is to assess the impact of ethyl propanoate modification on scaffold properties. This includes investigating how the compound affects the scaffold's porosity, mechanical strength, degradation rate, and ability to support cell adhesion and proliferation. Additionally, we aim to explore the potential synergistic effects between ethyl propanoate and other bioactive molecules commonly used in tissue engineering applications.
Another important objective is to identify the most suitable fabrication methods for incorporating ethyl propanoate into various scaffold materials. This involves evaluating different techniques such as electrospinning, 3D printing, and solvent casting, to determine which approaches yield the most effective and reproducible results. Furthermore, we seek to understand the optimal concentration and distribution of ethyl propanoate within the scaffold structure to maximize its beneficial effects.
As we delve into this emerging field, it is crucial to consider the broader implications of ethyl propanoate-modified scaffolds in the context of tissue engineering and regenerative medicine. This includes assessing their potential applications in specific tissue types, such as bone, cartilage, or soft tissues, and exploring their compatibility with various cell types and growth factors. By setting these comprehensive objectives, we aim to provide a thorough evaluation of the technology's potential and guide future research and development efforts in this promising area of tissue engineering.
Market Analysis for Modified Tissue Engineering Scaffolds
The market for modified tissue engineering scaffolds, particularly those incorporating ethyl propanoate, is experiencing significant growth and transformation. This sector is driven by the increasing demand for advanced biomaterials in regenerative medicine and tissue engineering applications. The global tissue engineering market, which encompasses modified scaffolds, is projected to expand at a robust rate over the next decade.
Key factors fueling market growth include the rising prevalence of chronic diseases, an aging population, and advancements in biomaterial technologies. Ethyl propanoate modified scaffolds are gaining attention due to their potential to enhance cell adhesion, proliferation, and differentiation, which are crucial for successful tissue regeneration.
The healthcare industry's shift towards personalized medicine and regenerative therapies is creating new opportunities for modified scaffold technologies. Hospitals, research institutions, and biotechnology companies are increasingly investing in tissue engineering solutions, driving demand for innovative scaffold materials.
Geographically, North America and Europe currently dominate the market for modified tissue engineering scaffolds, owing to their advanced healthcare infrastructure and substantial research funding. However, Asia-Pacific is emerging as a rapidly growing market, with countries like China, Japan, and South Korea making significant investments in regenerative medicine research and development.
The market for ethyl propanoate modified scaffolds is segmented based on application areas, including orthopedics, cardiovascular, dermatology, and neurology. Orthopedic applications, particularly for bone and cartilage regeneration, represent a substantial portion of the market share due to the high incidence of musculoskeletal disorders and injuries.
Competitive landscape analysis reveals that both established medical device companies and innovative startups are actively developing and commercializing modified scaffold technologies. Strategic collaborations between academic institutions and industry players are accelerating the translation of research findings into marketable products.
Regulatory considerations play a crucial role in market dynamics. The approval process for tissue engineering products, including modified scaffolds, can be complex and time-consuming, impacting market entry and product development timelines. However, regulatory bodies are increasingly recognizing the potential of these technologies and working to streamline approval processes.
Market challenges include high development and manufacturing costs, which can affect product pricing and accessibility. Additionally, ensuring consistent quality and performance of modified scaffolds across different batches remains a technical hurdle that manufacturers must address.
Despite these challenges, the market outlook for ethyl propanoate modified tissue engineering scaffolds remains positive. The growing body of clinical evidence supporting their efficacy, coupled with ongoing technological advancements, is expected to drive continued market expansion and innovation in the coming years.
Key factors fueling market growth include the rising prevalence of chronic diseases, an aging population, and advancements in biomaterial technologies. Ethyl propanoate modified scaffolds are gaining attention due to their potential to enhance cell adhesion, proliferation, and differentiation, which are crucial for successful tissue regeneration.
The healthcare industry's shift towards personalized medicine and regenerative therapies is creating new opportunities for modified scaffold technologies. Hospitals, research institutions, and biotechnology companies are increasingly investing in tissue engineering solutions, driving demand for innovative scaffold materials.
Geographically, North America and Europe currently dominate the market for modified tissue engineering scaffolds, owing to their advanced healthcare infrastructure and substantial research funding. However, Asia-Pacific is emerging as a rapidly growing market, with countries like China, Japan, and South Korea making significant investments in regenerative medicine research and development.
The market for ethyl propanoate modified scaffolds is segmented based on application areas, including orthopedics, cardiovascular, dermatology, and neurology. Orthopedic applications, particularly for bone and cartilage regeneration, represent a substantial portion of the market share due to the high incidence of musculoskeletal disorders and injuries.
Competitive landscape analysis reveals that both established medical device companies and innovative startups are actively developing and commercializing modified scaffold technologies. Strategic collaborations between academic institutions and industry players are accelerating the translation of research findings into marketable products.
Regulatory considerations play a crucial role in market dynamics. The approval process for tissue engineering products, including modified scaffolds, can be complex and time-consuming, impacting market entry and product development timelines. However, regulatory bodies are increasingly recognizing the potential of these technologies and working to streamline approval processes.
Market challenges include high development and manufacturing costs, which can affect product pricing and accessibility. Additionally, ensuring consistent quality and performance of modified scaffolds across different batches remains a technical hurdle that manufacturers must address.
Despite these challenges, the market outlook for ethyl propanoate modified tissue engineering scaffolds remains positive. The growing body of clinical evidence supporting their efficacy, coupled with ongoing technological advancements, is expected to drive continued market expansion and innovation in the coming years.
Current Challenges in Ethyl Propanoate Scaffold Development
The development of ethyl propanoate modified tissue engineering scaffolds faces several significant challenges that hinder their widespread adoption and clinical application. One of the primary obstacles is achieving optimal mechanical properties that closely mimic the native tissue environment. While ethyl propanoate modification can enhance certain scaffold characteristics, fine-tuning the balance between strength, flexibility, and degradation rate remains a complex task.
Biocompatibility and immunogenicity present another critical challenge. Although ethyl propanoate is generally considered safe, its incorporation into scaffolds may alter the immune response in vivo. Researchers must carefully evaluate potential inflammatory reactions and ensure long-term compatibility with host tissues. This requires extensive in vitro and in vivo testing, which can be time-consuming and resource-intensive.
Controlled degradation kinetics pose a significant hurdle in ethyl propanoate scaffold development. The ideal scaffold should degrade at a rate that matches new tissue formation, but achieving this delicate balance is challenging. Ethyl propanoate modification can affect degradation rates, and researchers must optimize this process to ensure proper tissue regeneration without compromising structural integrity prematurely.
Scalability and reproducibility of ethyl propanoate modified scaffolds represent substantial challenges for industrial production. Maintaining consistent quality and properties across large-scale manufacturing processes is crucial for clinical translation. Variations in ethyl propanoate incorporation and distribution within scaffolds can lead to inconsistent performance, necessitating robust quality control measures.
Another challenge lies in achieving uniform cell distribution and vascularization within ethyl propanoate modified scaffolds. While the modification can enhance certain aspects of cell adhesion and proliferation, ensuring even cell distribution throughout the scaffold and promoting adequate vascularization for nutrient supply remain ongoing concerns. This is particularly critical for larger, three-dimensional constructs intended for complex tissue engineering applications.
Regulatory hurdles and safety concerns also present significant challenges in the development of ethyl propanoate modified scaffolds. As a novel modification technique, regulatory agencies may require extensive safety data and clinical trials before approving such scaffolds for human use. This process can be lengthy and costly, potentially slowing down the translation of promising research into clinical applications.
Lastly, the integration of ethyl propanoate modified scaffolds with other tissue engineering technologies, such as growth factor delivery systems or cell encapsulation methods, presents complex challenges. Researchers must ensure that the ethyl propanoate modification does not interfere with these complementary approaches and may need to develop new strategies for combining multiple technologies effectively.
Biocompatibility and immunogenicity present another critical challenge. Although ethyl propanoate is generally considered safe, its incorporation into scaffolds may alter the immune response in vivo. Researchers must carefully evaluate potential inflammatory reactions and ensure long-term compatibility with host tissues. This requires extensive in vitro and in vivo testing, which can be time-consuming and resource-intensive.
Controlled degradation kinetics pose a significant hurdle in ethyl propanoate scaffold development. The ideal scaffold should degrade at a rate that matches new tissue formation, but achieving this delicate balance is challenging. Ethyl propanoate modification can affect degradation rates, and researchers must optimize this process to ensure proper tissue regeneration without compromising structural integrity prematurely.
Scalability and reproducibility of ethyl propanoate modified scaffolds represent substantial challenges for industrial production. Maintaining consistent quality and properties across large-scale manufacturing processes is crucial for clinical translation. Variations in ethyl propanoate incorporation and distribution within scaffolds can lead to inconsistent performance, necessitating robust quality control measures.
Another challenge lies in achieving uniform cell distribution and vascularization within ethyl propanoate modified scaffolds. While the modification can enhance certain aspects of cell adhesion and proliferation, ensuring even cell distribution throughout the scaffold and promoting adequate vascularization for nutrient supply remain ongoing concerns. This is particularly critical for larger, three-dimensional constructs intended for complex tissue engineering applications.
Regulatory hurdles and safety concerns also present significant challenges in the development of ethyl propanoate modified scaffolds. As a novel modification technique, regulatory agencies may require extensive safety data and clinical trials before approving such scaffolds for human use. This process can be lengthy and costly, potentially slowing down the translation of promising research into clinical applications.
Lastly, the integration of ethyl propanoate modified scaffolds with other tissue engineering technologies, such as growth factor delivery systems or cell encapsulation methods, presents complex challenges. Researchers must ensure that the ethyl propanoate modification does not interfere with these complementary approaches and may need to develop new strategies for combining multiple technologies effectively.
Existing Ethyl Propanoate Scaffold Modification Techniques
01 Surface modification of scaffolds
Surface modification techniques are employed to enhance the biocompatibility and cell adhesion properties of tissue engineering scaffolds. This can involve chemical treatments, plasma processing, or coating with bioactive molecules to improve cell attachment, proliferation, and differentiation on the scaffold surface.- Surface modification of scaffolds: Surface modification techniques are employed to enhance the biocompatibility and functionality of tissue engineering scaffolds. These methods can include chemical treatments, plasma processing, or coating with bioactive molecules to improve cell adhesion, proliferation, and differentiation. Such modifications can significantly influence the scaffold's interaction with cells and surrounding tissues.
- Incorporation of growth factors and biomolecules: Tissue engineering scaffolds can be modified by incorporating growth factors, proteins, and other biomolecules. This approach aims to create a more biomimetic environment that promotes tissue regeneration and cellular activities. The controlled release of these bioactive molecules from the scaffold can guide tissue formation and enhance the overall regenerative process.
- Nanostructure integration in scaffolds: Integrating nanostructures into tissue engineering scaffolds is an emerging approach to mimic the natural extracellular matrix more closely. This can involve the use of nanofibers, nanoparticles, or other nanoscale features to enhance the scaffold's mechanical properties, increase surface area, and provide topographical cues for cell guidance and tissue organization.
- Smart and stimuli-responsive scaffolds: Development of smart and stimuli-responsive scaffolds involves modifying the scaffold material to respond to external stimuli such as temperature, pH, or electrical signals. These advanced scaffolds can change their properties on-demand, allowing for dynamic control over cell behavior, drug release, or scaffold degradation, which can be beneficial for various tissue engineering applications.
- Biodegradable and composite scaffold materials: Modification of scaffold materials to improve their biodegradability and mechanical properties is a key area of research. This often involves creating composite materials that combine synthetic and natural polymers or incorporating inorganic components. Such modifications aim to achieve an optimal balance between scaffold degradation rate and tissue regeneration, while also providing appropriate mechanical support.
02 Incorporation of growth factors and bioactive agents
Tissue engineering scaffolds can be modified by incorporating growth factors, cytokines, and other bioactive agents. These molecules can be encapsulated within the scaffold material or attached to the surface to promote tissue regeneration, angiogenesis, and cell differentiation.Expand Specific Solutions03 Nanostructure modification
Nanostructure modification of scaffolds involves altering the scaffold's surface at the nanoscale level. This can include creating nanopatterns, incorporating nanoparticles, or developing nanofiber structures to mimic the natural extracellular matrix and enhance cell-scaffold interactions.Expand Specific Solutions04 Biodegradable and stimuli-responsive scaffolds
Development of biodegradable and stimuli-responsive scaffolds that can change their properties in response to external stimuli such as pH, temperature, or enzymatic activity. These modifications allow for controlled degradation rates and release of encapsulated bioactive agents, enhancing tissue regeneration.Expand Specific Solutions05 3D printing and additive manufacturing techniques
Utilization of 3D printing and additive manufacturing techniques to create custom-designed scaffolds with precise geometries and controlled porosity. These methods allow for the fabrication of patient-specific scaffolds and the incorporation of multiple materials or bioactive agents within a single construct.Expand Specific Solutions
Key Players in Modified Scaffold Research and Development
The field of ethyl propanoate modified tissue engineering scaffolds is in an early developmental stage, with significant potential for growth. The market size is currently modest but expected to expand as research progresses and applications emerge. Technologically, the field is still maturing, with ongoing research at institutions like Drexel University, University of South Florida, and Southeast University. Companies such as Coloplast A/S and Collagen Solutions NZ Ltd. are exploring commercial applications, indicating growing industry interest. However, the technology's full potential and market impact remain to be fully realized, suggesting a dynamic competitive landscape with opportunities for both academic and industrial players to make significant contributions.
Drexel University
Technical Solution: Drexel University has developed a novel approach to tissue engineering scaffolds modified with ethyl propanoate. Their research focuses on improving the biocompatibility and mechanical properties of scaffolds. The university has created a proprietary process for incorporating ethyl propanoate into various scaffold materials, including polymers and hydrogels. This modification enhances cell adhesion and proliferation, while also improving the scaffold's degradation profile[1]. Drexel's method involves a controlled release mechanism of ethyl propanoate, which stimulates tissue regeneration and vascularization. The university has also explored the use of 3D printing techniques to create custom-shaped scaffolds with precise ethyl propanoate distribution[3].
Strengths: Enhanced cell adhesion and proliferation, improved degradation profile, and customizable scaffold shapes. Weaknesses: Potential long-term effects of ethyl propanoate on tissue development still under investigation.
Technion Research & Development Foundation Ltd.
Technical Solution: Technion has developed an innovative approach to ethyl propanoate-modified tissue engineering scaffolds. Their research focuses on creating nanocomposite scaffolds that incorporate ethyl propanoate into biodegradable polymers. The foundation has engineered a unique process that allows for controlled release of ethyl propanoate, enhancing cell growth and differentiation[2]. Technion's scaffolds utilize a gradient distribution of ethyl propanoate, which mimics the natural extracellular matrix more closely. They have also developed a novel surface modification technique that increases the scaffold's hydrophilicity, improving cell attachment and proliferation[4]. Additionally, Technion has explored the use of ethyl propanoate in conjunction with growth factors to create synergistic effects on tissue regeneration.
Strengths: Controlled release of ethyl propanoate, gradient distribution mimicking natural ECM, and improved hydrophilicity. Weaknesses: Potential scalability issues for large-scale production and long-term stability of the nanocomposite structure.
Innovative Approaches in Ethyl Propanoate Scaffold Design
Fruity odorant
PatentActiveUS20110097291A1
Innovation
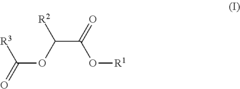
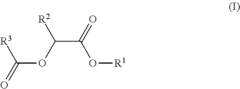
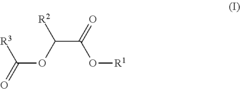
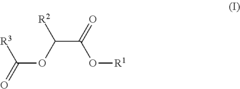
- Development of di-ester compounds of formula (I), specifically C8 or C10 compounds with specific alkyl and alkenyl groups, which can be used as perfuming ingredients to impart fruity-apricot or fruity-vinous notes without herbaceous characteristics, and their incorporation into perfuming compositions or articles.
Flavor composition or fragrance composition, product containing the flavor composition or fragrance composition, and novel ester compound
PatentInactiveEP2098586B1
Innovation
- The development of carboxylic acid esters of 2-methyl-2-pentenol with formic acid or carboxylic acids having 1 to 9 carbon atoms, which exhibit unprecedented fruity, greenish, and floral aromas and flavors, offering a new type of aroma and flavor profile.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when evaluating ethyl propanoate modified tissue engineering scaffolds. The introduction of ethyl propanoate as a modifying agent necessitates a comprehensive assessment of its potential impact on cellular interactions, tissue integration, and overall patient safety.
One of the primary concerns is the potential cytotoxicity of ethyl propanoate and its degradation products. In vitro studies using relevant cell lines must be conducted to evaluate cell viability, proliferation, and morphology when exposed to the modified scaffolds. These tests should include both direct contact assays and extract-based methods to assess any leachable components that may affect cell health.
The inflammatory response to ethyl propanoate modified scaffolds is another critical aspect to investigate. Immune cell activation and pro-inflammatory cytokine production should be measured to ensure that the scaffolds do not elicit an excessive or prolonged inflammatory reaction. This is particularly important for long-term implantation scenarios where chronic inflammation could lead to fibrosis or implant rejection.
Genotoxicity and mutagenicity assessments are essential to rule out any potential DNA damage or carcinogenic effects of the modified scaffolds. Standard in vitro and in vivo tests, such as the Ames test and chromosomal aberration assays, should be performed to evaluate the genetic safety of the material.
The biodegradation profile of ethyl propanoate modified scaffolds must be carefully characterized. The rate of degradation, the nature of degradation products, and their potential systemic effects need to be thoroughly investigated. This includes evaluating the pH changes in the local microenvironment during degradation and assessing the impact on surrounding tissues.
Hemocompatibility is another crucial factor, especially for scaffolds intended for use in vascular or blood-contacting applications. Tests for hemolysis, platelet activation, and thrombogenicity should be conducted to ensure that the modified scaffolds do not adversely affect blood components or promote clot formation.
Long-term biocompatibility studies in animal models are indispensable for predicting the in vivo performance of ethyl propanoate modified scaffolds. These studies should assess tissue integration, vascularization, and any potential systemic toxicity over extended periods. Histological analysis of explanted scaffolds and surrounding tissues can provide valuable insights into the host response and tissue remodeling processes.
Sterilization compatibility is an often-overlooked aspect of scaffold safety. The impact of various sterilization methods on the physical and chemical properties of ethyl propanoate modified scaffolds must be evaluated to ensure that the sterilization process does not compromise the scaffold's integrity or introduce harmful byproducts.
One of the primary concerns is the potential cytotoxicity of ethyl propanoate and its degradation products. In vitro studies using relevant cell lines must be conducted to evaluate cell viability, proliferation, and morphology when exposed to the modified scaffolds. These tests should include both direct contact assays and extract-based methods to assess any leachable components that may affect cell health.
The inflammatory response to ethyl propanoate modified scaffolds is another critical aspect to investigate. Immune cell activation and pro-inflammatory cytokine production should be measured to ensure that the scaffolds do not elicit an excessive or prolonged inflammatory reaction. This is particularly important for long-term implantation scenarios where chronic inflammation could lead to fibrosis or implant rejection.
Genotoxicity and mutagenicity assessments are essential to rule out any potential DNA damage or carcinogenic effects of the modified scaffolds. Standard in vitro and in vivo tests, such as the Ames test and chromosomal aberration assays, should be performed to evaluate the genetic safety of the material.
The biodegradation profile of ethyl propanoate modified scaffolds must be carefully characterized. The rate of degradation, the nature of degradation products, and their potential systemic effects need to be thoroughly investigated. This includes evaluating the pH changes in the local microenvironment during degradation and assessing the impact on surrounding tissues.
Hemocompatibility is another crucial factor, especially for scaffolds intended for use in vascular or blood-contacting applications. Tests for hemolysis, platelet activation, and thrombogenicity should be conducted to ensure that the modified scaffolds do not adversely affect blood components or promote clot formation.
Long-term biocompatibility studies in animal models are indispensable for predicting the in vivo performance of ethyl propanoate modified scaffolds. These studies should assess tissue integration, vascularization, and any potential systemic toxicity over extended periods. Histological analysis of explanted scaffolds and surrounding tissues can provide valuable insights into the host response and tissue remodeling processes.
Sterilization compatibility is an often-overlooked aspect of scaffold safety. The impact of various sterilization methods on the physical and chemical properties of ethyl propanoate modified scaffolds must be evaluated to ensure that the sterilization process does not compromise the scaffold's integrity or introduce harmful byproducts.
Regulatory Pathway for Novel Tissue Engineering Scaffolds
The regulatory pathway for novel tissue engineering scaffolds incorporating ethyl propanoate modifications requires careful navigation of existing frameworks and potential adaptations to address the unique characteristics of these innovative materials. The U.S. Food and Drug Administration (FDA) classifies most tissue engineering scaffolds as combination products, typically regulated under the medical device pathway with additional considerations for biological components.
For ethyl propanoate modified scaffolds, manufacturers would likely need to submit a premarket approval (PMA) application or a 510(k) premarket notification, depending on the level of risk and similarity to predicate devices. The choice between these pathways would be influenced by the specific modifications introduced by ethyl propanoate and their impact on the scaffold's safety and efficacy profile.
A critical aspect of the regulatory process will be demonstrating the safety of ethyl propanoate as a novel component in tissue engineering scaffolds. This may require extensive toxicology studies and biocompatibility testing to assess any potential adverse effects on cellular interactions, tissue regeneration, and long-term patient outcomes. Manufacturers should engage in early discussions with the FDA through the Q-Submission program to seek guidance on the appropriate testing protocols and data requirements.
The regulatory pathway will also necessitate comprehensive characterization of the ethyl propanoate modified scaffolds, including their physical, chemical, and biological properties. This characterization should address how the ethyl propanoate modification affects the scaffold's degradation profile, mechanical properties, and ability to support tissue growth and integration.
Clinical evaluation strategies for these novel scaffolds may need to be tailored to address specific regulatory concerns. This could involve designing clinical trials that not only demonstrate efficacy but also provide long-term safety data on the ethyl propanoate component. The FDA may require post-market surveillance studies to monitor the performance and safety of these scaffolds over extended periods.
Manufacturers should also consider international regulatory requirements, particularly if seeking global market access. The European Union's Medical Device Regulation (MDR) and other regional frameworks may have different classification systems and evidence requirements for novel tissue engineering scaffolds.
As the field of tissue engineering continues to evolve, regulatory agencies may need to adapt their approaches to accommodate innovative materials like ethyl propanoate modified scaffolds. This could lead to the development of new guidance documents or regulatory pathways specifically tailored to advanced tissue engineering products. Staying informed about these potential regulatory changes and actively engaging with regulatory bodies will be crucial for successful commercialization of these novel scaffolds.
For ethyl propanoate modified scaffolds, manufacturers would likely need to submit a premarket approval (PMA) application or a 510(k) premarket notification, depending on the level of risk and similarity to predicate devices. The choice between these pathways would be influenced by the specific modifications introduced by ethyl propanoate and their impact on the scaffold's safety and efficacy profile.
A critical aspect of the regulatory process will be demonstrating the safety of ethyl propanoate as a novel component in tissue engineering scaffolds. This may require extensive toxicology studies and biocompatibility testing to assess any potential adverse effects on cellular interactions, tissue regeneration, and long-term patient outcomes. Manufacturers should engage in early discussions with the FDA through the Q-Submission program to seek guidance on the appropriate testing protocols and data requirements.
The regulatory pathway will also necessitate comprehensive characterization of the ethyl propanoate modified scaffolds, including their physical, chemical, and biological properties. This characterization should address how the ethyl propanoate modification affects the scaffold's degradation profile, mechanical properties, and ability to support tissue growth and integration.
Clinical evaluation strategies for these novel scaffolds may need to be tailored to address specific regulatory concerns. This could involve designing clinical trials that not only demonstrate efficacy but also provide long-term safety data on the ethyl propanoate component. The FDA may require post-market surveillance studies to monitor the performance and safety of these scaffolds over extended periods.
Manufacturers should also consider international regulatory requirements, particularly if seeking global market access. The European Union's Medical Device Regulation (MDR) and other regional frameworks may have different classification systems and evidence requirements for novel tissue engineering scaffolds.
As the field of tissue engineering continues to evolve, regulatory agencies may need to adapt their approaches to accommodate innovative materials like ethyl propanoate modified scaffolds. This could lead to the development of new guidance documents or regulatory pathways specifically tailored to advanced tissue engineering products. Staying informed about these potential regulatory changes and actively engaging with regulatory bodies will be crucial for successful commercialization of these novel scaffolds.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!