Ammonia cracking assisted by hydrogen membrane reactors
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Cracking Technology Evolution and Objectives
Ammonia has emerged as a promising hydrogen carrier due to its high hydrogen content (17.6% by weight) and established infrastructure for production, storage, and transportation. The evolution of ammonia cracking technology represents a critical pathway in the global transition toward hydrogen-based clean energy systems. Historically, ammonia decomposition has been studied since the early 20th century, primarily as a chemical process rather than an energy application.
The technological trajectory began with conventional thermal catalytic cracking methods requiring high temperatures (>450°C) and energy-intensive operations. These early systems suffered from low efficiency and significant energy penalties, making them impractical for widespread hydrogen delivery applications. The introduction of structured catalysts in the 1980s marked an important advancement, improving reaction kinetics while maintaining operational challenges.
A paradigm shift occurred in the early 2000s with the integration of membrane technology into ammonia cracking systems. This innovation addressed the thermodynamic limitations of conventional approaches by selectively removing hydrogen from the reaction zone, thereby shifting equilibrium toward complete ammonia conversion at lower temperatures. The membrane-assisted approach represents a critical evolutionary step, potentially reducing energy requirements by 20-30% compared to conventional methods.
Recent technological developments have focused on novel catalyst formulations, particularly ruthenium and nickel-based systems, that demonstrate enhanced activity and stability. Parallel advancements in membrane materials, especially palladium-based alloys and ceramic proton conductors, have significantly improved hydrogen separation efficiency and operational durability under ammonia cracking conditions.
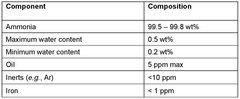
The primary objectives driving ammonia cracking technology development include achieving complete ammonia conversion at temperatures below 400°C, minimizing energy input requirements, ensuring catalyst longevity exceeding 5,000 operating hours, and developing compact reactor designs suitable for distributed applications. Additionally, there is growing emphasis on process intensification through multifunctional reactor concepts that combine reaction and separation in single units.
Looking forward, the technology roadmap aims to enable efficient hydrogen recovery from ammonia at scales ranging from kilowatts to megawatts, supporting applications from fuel cell vehicles to grid-scale energy storage. The ultimate goal is establishing ammonia as a viable hydrogen vector within a circular nitrogen-hydrogen economy, where ammonia serves as both an energy carrier and a renewable chemical feedstock.
The technological trajectory began with conventional thermal catalytic cracking methods requiring high temperatures (>450°C) and energy-intensive operations. These early systems suffered from low efficiency and significant energy penalties, making them impractical for widespread hydrogen delivery applications. The introduction of structured catalysts in the 1980s marked an important advancement, improving reaction kinetics while maintaining operational challenges.
A paradigm shift occurred in the early 2000s with the integration of membrane technology into ammonia cracking systems. This innovation addressed the thermodynamic limitations of conventional approaches by selectively removing hydrogen from the reaction zone, thereby shifting equilibrium toward complete ammonia conversion at lower temperatures. The membrane-assisted approach represents a critical evolutionary step, potentially reducing energy requirements by 20-30% compared to conventional methods.
Recent technological developments have focused on novel catalyst formulations, particularly ruthenium and nickel-based systems, that demonstrate enhanced activity and stability. Parallel advancements in membrane materials, especially palladium-based alloys and ceramic proton conductors, have significantly improved hydrogen separation efficiency and operational durability under ammonia cracking conditions.
The primary objectives driving ammonia cracking technology development include achieving complete ammonia conversion at temperatures below 400°C, minimizing energy input requirements, ensuring catalyst longevity exceeding 5,000 operating hours, and developing compact reactor designs suitable for distributed applications. Additionally, there is growing emphasis on process intensification through multifunctional reactor concepts that combine reaction and separation in single units.
Looking forward, the technology roadmap aims to enable efficient hydrogen recovery from ammonia at scales ranging from kilowatts to megawatts, supporting applications from fuel cell vehicles to grid-scale energy storage. The ultimate goal is establishing ammonia as a viable hydrogen vector within a circular nitrogen-hydrogen economy, where ammonia serves as both an energy carrier and a renewable chemical feedstock.
Market Analysis for Hydrogen Production via Ammonia Cracking
The global hydrogen market is experiencing significant growth, driven by increasing focus on decarbonization and clean energy transitions. Currently valued at approximately $130 billion, the hydrogen market is projected to reach $500 billion by 2030, with annual growth rates exceeding 9.2% through the decade. Within this expanding market, hydrogen production via ammonia cracking represents a particularly promising segment due to its potential to address key challenges in hydrogen transportation and storage.
Ammonia (NH3) serves as an efficient hydrogen carrier, containing 17.6% hydrogen by weight and offering significantly higher volumetric hydrogen density compared to compressed or liquid hydrogen. This characteristic makes ammonia-based hydrogen delivery systems increasingly attractive for long-distance transport scenarios where direct hydrogen pipelines are impractical or cost-prohibitive.
The demand for hydrogen from ammonia cracking is being driven by several key market sectors. The industrial sector, particularly refineries and chemical manufacturing, represents the largest current market, accounting for approximately 72% of hydrogen consumption. However, emerging applications in transportation (fuel cell vehicles), power generation, and residential heating are expected to dramatically reshape demand patterns over the next decade.
Regionally, Asia-Pacific dominates the market for ammonia cracking technologies, with Japan and South Korea leading adoption due to national hydrogen strategy commitments. Europe follows closely, with Germany, Netherlands, and Nordic countries investing heavily in ammonia import terminals equipped with cracking facilities. North America shows growing interest, particularly in industrial clusters along coastal regions.
Market analysis indicates that membrane reactor technologies for ammonia cracking could capture 25-30% of the hydrogen production technology market by 2035, representing a significant opportunity for early movers. The cost trajectory is favorable, with production costs expected to decrease from current $4-5/kg to potentially $2-3/kg by 2030 as technologies mature and achieve scale.
Key market drivers include increasingly stringent carbon regulations, renewable energy integration challenges requiring chemical storage solutions, and industrial decarbonization initiatives. The shipping industry represents a particularly promising growth segment, with ammonia being considered both as a direct fuel and as a hydrogen carrier for maritime applications.
Market barriers include competition from alternative hydrogen production methods (particularly electrolysis), high initial capital requirements for cracking facilities, and technical challenges in membrane durability and efficiency. Additionally, safety concerns and regulatory frameworks for ammonia handling infrastructure remain important market development considerations.
Ammonia (NH3) serves as an efficient hydrogen carrier, containing 17.6% hydrogen by weight and offering significantly higher volumetric hydrogen density compared to compressed or liquid hydrogen. This characteristic makes ammonia-based hydrogen delivery systems increasingly attractive for long-distance transport scenarios where direct hydrogen pipelines are impractical or cost-prohibitive.
The demand for hydrogen from ammonia cracking is being driven by several key market sectors. The industrial sector, particularly refineries and chemical manufacturing, represents the largest current market, accounting for approximately 72% of hydrogen consumption. However, emerging applications in transportation (fuel cell vehicles), power generation, and residential heating are expected to dramatically reshape demand patterns over the next decade.
Regionally, Asia-Pacific dominates the market for ammonia cracking technologies, with Japan and South Korea leading adoption due to national hydrogen strategy commitments. Europe follows closely, with Germany, Netherlands, and Nordic countries investing heavily in ammonia import terminals equipped with cracking facilities. North America shows growing interest, particularly in industrial clusters along coastal regions.
Market analysis indicates that membrane reactor technologies for ammonia cracking could capture 25-30% of the hydrogen production technology market by 2035, representing a significant opportunity for early movers. The cost trajectory is favorable, with production costs expected to decrease from current $4-5/kg to potentially $2-3/kg by 2030 as technologies mature and achieve scale.
Key market drivers include increasingly stringent carbon regulations, renewable energy integration challenges requiring chemical storage solutions, and industrial decarbonization initiatives. The shipping industry represents a particularly promising growth segment, with ammonia being considered both as a direct fuel and as a hydrogen carrier for maritime applications.
Market barriers include competition from alternative hydrogen production methods (particularly electrolysis), high initial capital requirements for cracking facilities, and technical challenges in membrane durability and efficiency. Additionally, safety concerns and regulatory frameworks for ammonia handling infrastructure remain important market development considerations.
Technical Barriers in Membrane-Assisted Ammonia Decomposition
Despite significant advancements in membrane-assisted ammonia decomposition technology, several critical technical barriers continue to impede widespread commercial implementation. The primary challenge remains membrane stability under the harsh operating conditions required for ammonia cracking. Current membrane materials, particularly palladium-based systems, suffer from hydrogen embrittlement and sulfur poisoning, significantly reducing their operational lifespan in industrial settings. Even trace contaminants in ammonia feedstock can lead to rapid performance degradation.
Thermal management presents another substantial hurdle. The endothermic nature of ammonia decomposition requires consistent heat input, yet temperature gradients across membrane reactors often lead to uneven reaction rates and membrane stress. This thermal cycling accelerates membrane fatigue and creates localized hotspots that compromise structural integrity, particularly at the membrane-support interface where thermal expansion coefficients may differ.
Membrane selectivity remains suboptimal, with current technologies struggling to achieve the 99.99+% hydrogen purity required for certain applications like PEM fuel cells. Cross-contamination with ammonia, nitrogen, and other trace gases necessitates additional downstream purification steps, reducing overall system efficiency and increasing complexity.
Scale-up challenges persist in translating laboratory success to industrial implementation. The fabrication of defect-free membranes with consistent properties becomes increasingly difficult at commercial scales. Current manufacturing techniques cannot reliably produce large-area membranes with uniform thickness and performance characteristics, resulting in significant batch-to-batch variations.
Economic barriers compound these technical challenges. The high cost of membrane materials, particularly those utilizing precious metals like palladium, makes large-scale deployment prohibitively expensive. While alternative materials show promise in laboratory settings, they typically sacrifice either permeability or selectivity, creating an unfavorable performance-cost tradeoff.
Catalyst integration with membrane systems introduces additional complexities. Optimal catalyst positioning relative to the membrane surface remains difficult to standardize, and catalyst deactivation pathways are accelerated in the membrane reactor environment. The synergistic effects between catalyst and membrane materials are not fully understood, limiting rational design approaches.
System integration challenges further complicate implementation, as membrane reactors must interface with existing ammonia production or utilization infrastructure. Pressure differentials across the membrane must be carefully managed to prevent mechanical failure while maintaining sufficient driving force for hydrogen permeation, requiring sophisticated control systems that add cost and complexity.
Thermal management presents another substantial hurdle. The endothermic nature of ammonia decomposition requires consistent heat input, yet temperature gradients across membrane reactors often lead to uneven reaction rates and membrane stress. This thermal cycling accelerates membrane fatigue and creates localized hotspots that compromise structural integrity, particularly at the membrane-support interface where thermal expansion coefficients may differ.
Membrane selectivity remains suboptimal, with current technologies struggling to achieve the 99.99+% hydrogen purity required for certain applications like PEM fuel cells. Cross-contamination with ammonia, nitrogen, and other trace gases necessitates additional downstream purification steps, reducing overall system efficiency and increasing complexity.
Scale-up challenges persist in translating laboratory success to industrial implementation. The fabrication of defect-free membranes with consistent properties becomes increasingly difficult at commercial scales. Current manufacturing techniques cannot reliably produce large-area membranes with uniform thickness and performance characteristics, resulting in significant batch-to-batch variations.
Economic barriers compound these technical challenges. The high cost of membrane materials, particularly those utilizing precious metals like palladium, makes large-scale deployment prohibitively expensive. While alternative materials show promise in laboratory settings, they typically sacrifice either permeability or selectivity, creating an unfavorable performance-cost tradeoff.
Catalyst integration with membrane systems introduces additional complexities. Optimal catalyst positioning relative to the membrane surface remains difficult to standardize, and catalyst deactivation pathways are accelerated in the membrane reactor environment. The synergistic effects between catalyst and membrane materials are not fully understood, limiting rational design approaches.
System integration challenges further complicate implementation, as membrane reactors must interface with existing ammonia production or utilization infrastructure. Pressure differentials across the membrane must be carefully managed to prevent mechanical failure while maintaining sufficient driving force for hydrogen permeation, requiring sophisticated control systems that add cost and complexity.
Current Membrane Reactor Configurations for Ammonia Processing
01 Membrane materials for hydrogen separation in ammonia cracking
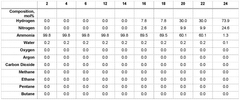
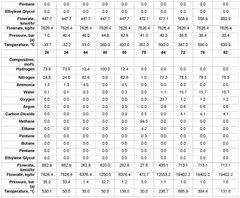
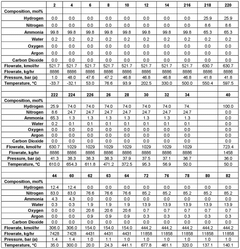
Various membrane materials are used in hydrogen membrane reactors for ammonia cracking to efficiently separate hydrogen from other gases. These materials include palladium-based membranes, ceramic membranes, and composite membranes that offer high hydrogen permeability and selectivity. The choice of membrane material significantly affects the efficiency of hydrogen separation and the overall performance of the ammonia cracking process. Advanced membrane materials can withstand high temperatures and harsh conditions typically present during ammonia decomposition.- Membrane reactor design for ammonia cracking: Specialized membrane reactor designs can enhance ammonia cracking efficiency by integrating hydrogen separation with the decomposition reaction. These reactors typically feature palladium or palladium-alloy membranes that selectively allow hydrogen to permeate while retaining nitrogen and unreacted ammonia. The continuous removal of hydrogen shifts the reaction equilibrium toward higher ammonia conversion rates, improving overall efficiency and hydrogen yield. Various configurations include tubular, planar, and multi-channel designs optimized for heat transfer and reaction kinetics.
- Catalyst systems for ammonia decomposition: Advanced catalyst systems play a crucial role in ammonia cracking efficiency within membrane reactors. Ruthenium-based catalysts are particularly effective due to their high activity for N-H bond breaking, while nickel-based catalysts offer cost advantages. Catalyst performance can be enhanced through promoters like potassium or cesium, and support materials such as alumina or carbon nanotubes that increase surface area and stability. Structured catalyst configurations, including monoliths and microchannels, improve mass transfer and reduce pressure drop across the reactor system.
- Hydrogen separation and purification techniques: Effective hydrogen separation is essential in ammonia cracking membrane reactors. Various membrane materials and configurations are employed, including dense metallic membranes (particularly palladium-based), ceramic proton conductors, and composite structures. The separation efficiency depends on membrane thickness, operating temperature, pressure differential, and surface properties. Advanced techniques include surface modification to prevent poisoning, multi-layer composite membranes for enhanced durability, and module designs that maximize hydrogen flux while minimizing membrane area requirements.
- Thermal management and heat integration: Ammonia cracking is an endothermic reaction requiring significant heat input for efficient operation. Innovative thermal management approaches include heat integration with exothermic processes, microwave-assisted heating, structured heat exchangers, and catalytic combustion of byproducts. Advanced reactor designs incorporate heat transfer elements directly into the reaction zone to maintain optimal temperature profiles. Some systems utilize waste heat recovery to improve overall energy efficiency, while others employ staged heating to match the thermal requirements at different conversion levels.
- Process intensification and system integration: Process intensification strategies for ammonia cracking membrane reactors focus on combining multiple functions into compact, efficient units. Approaches include microreactor technology, modular designs for scalability, and integration with hydrogen storage or fuel cell systems. Advanced control systems optimize operating parameters in real-time based on demand fluctuations. Complete hydrogen production systems may integrate ammonia storage, vaporization, cracking, purification, and utilization components in a single package, particularly for distributed or mobile applications where space and weight constraints are significant.
02 Reactor design configurations for ammonia cracking
Innovative reactor designs enhance the efficiency of ammonia cracking processes in hydrogen membrane reactors. These designs include multi-stage reactors, microreactor arrays, and integrated heat exchange systems that optimize reaction conditions and hydrogen separation. Some configurations incorporate catalytic membrane reactors where the membrane serves both as a separator and reaction surface. The physical arrangement of catalysts, membranes, and flow channels within the reactor significantly impacts conversion rates and energy efficiency.Expand Specific Solutions03 Catalysts for enhanced ammonia decomposition
Specialized catalysts are crucial for efficient ammonia cracking in membrane reactor systems. These include ruthenium-based catalysts, nickel-based catalysts, and novel bimetallic formulations that lower the activation energy required for ammonia decomposition. Some catalysts are designed with specific support materials to increase surface area and stability at high temperatures. Advanced catalyst designs incorporate nanostructured materials that maximize active sites and improve reaction kinetics, leading to higher hydrogen yields at lower operating temperatures.Expand Specific Solutions04 Process integration and system optimization
Integrated approaches to ammonia cracking systems combine membrane reactors with other process units to optimize overall efficiency. These systems may include heat recovery mechanisms, pressure swing adsorption units, and integrated storage solutions. Process integration strategies focus on minimizing energy consumption while maximizing hydrogen recovery rates. Some designs incorporate renewable energy sources to power the endothermic ammonia cracking reaction, creating sustainable hydrogen production pathways. Advanced control systems monitor and adjust operating parameters in real-time to maintain optimal performance.Expand Specific Solutions05 Novel hydrogen purification techniques
Advanced purification techniques are employed in conjunction with membrane reactors to achieve ultra-high purity hydrogen from ammonia cracking. These techniques include multi-stage membrane separation, temperature swing processes, and hybrid separation systems combining membranes with other purification methods. Some approaches utilize selective adsorbents to remove trace contaminants from hydrogen streams. Novel purification strategies focus on reducing energy requirements while meeting stringent purity specifications for applications such as fuel cells and semiconductor manufacturing.Expand Specific Solutions
Industry Leaders in Ammonia Cracking and Membrane Systems
The ammonia cracking hydrogen membrane reactor market is in an early growth phase, characterized by increasing R&D investments and emerging commercial applications. The global market is projected to expand significantly as hydrogen economy develops, driven by decarbonization initiatives. Technologically, the field shows moderate maturity with several key players advancing membrane reactor designs. Industry leaders like Air Liquide, Air Products & Chemicals, and Johnson Matthey possess established expertise in industrial gas processing and catalysis, while thyssenkrupp and Topsoe are developing specialized ammonia cracking technologies. Academic institutions including Nanjing Tech University and University of South Carolina are contributing fundamental research. Chinese entities like Sinopec and Fuda Zijin are increasingly active, reflecting the strategic importance of this technology for energy transition applications.
Air Products & Chemicals, Inc.
Technical Solution: Air Products has developed advanced ammonia cracking technology integrated with hydrogen membrane reactors to efficiently produce high-purity hydrogen. Their system utilizes palladium-based membrane reactors that simultaneously perform ammonia decomposition and hydrogen separation. The process operates at temperatures between 400-550°C with specialized ruthenium-based catalysts that enhance conversion efficiency. Their membrane reactor design features a tubular configuration with the catalyst packed in the annular space between the membrane and the reactor wall, allowing for continuous hydrogen extraction through the membrane as it forms. This shifts the reaction equilibrium toward higher ammonia conversion rates, achieving over 99% conversion in a single pass. The company has also developed heat integration systems that recover thermal energy from the process to preheat the ammonia feed, significantly improving overall energy efficiency by reducing external heating requirements by approximately 25%.
Strengths: Achieves near-complete ammonia conversion in a single pass; produces ultra-high purity hydrogen suitable for fuel cells; integrated heat recovery systems improve energy efficiency. Weaknesses: Requires precious metal catalysts and membrane materials, increasing capital costs; membrane durability under industrial conditions remains a challenge; system requires precise temperature control to prevent catalyst deactivation.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has pioneered membrane reactor technology for ammonia cracking that combines their proprietary catalysts with palladium-silver alloy membranes. Their system operates at moderate temperatures (450-500°C) and utilizes specially formulated ruthenium catalysts supported on structured alumina carriers that maximize surface area while minimizing pressure drop. The membrane reactor design incorporates thin-film Pd-Ag membranes (3-5 μm thickness) deposited on porous stainless steel supports, providing exceptional hydrogen permeability while maintaining mechanical integrity. This configuration enables simultaneous reaction and separation, driving ammonia conversion beyond thermodynamic equilibrium limitations to achieve conversion rates exceeding 95% at lower temperatures than conventional systems. Johnson Matthey's technology also features modular designs that can be scaled according to hydrogen production requirements, with units capable of producing 50-500 kg H₂/day from ammonia. Their latest generation incorporates sulfur-tolerant catalyst formulations that maintain activity even with trace impurities in the ammonia feedstock.
Strengths: High-performance proprietary catalysts with extended lifetime; thin-film membrane technology offers excellent hydrogen flux rates; modular design allows for flexible deployment and scaling. Weaknesses: Membrane fabrication requires sophisticated manufacturing techniques, increasing costs; potential for membrane embrittlement over extended operation; system requires careful startup and shutdown procedures to preserve catalyst and membrane integrity.
Breakthrough Patents in Hydrogen-Selective Membrane Materials
Process and Apparatus for Cracking Ammonia
PatentPendingUS20240400382A1
Innovation
- A process that splits the ammonia cracking duty between a fired reactor and an electrically heated reactor, utilizing a first catalyst in the fired reactor at super-atmospheric pressure and a second catalyst in the electrically heated reactor, with the offgas from hydrogen recovery used as fuel in the fired reactor to optimize energy use and reduce fossil fuel dependency.
Process and apparatus for cracking ammonia
PatentWO2024253893A1
Innovation
- A process and apparatus that splits the ammonia cracking duty between a fired reactor and an electrically heated reactor, utilizing a first catalyst in the fired reactor and a second catalyst in the electrically heated reactor, where the offgas from hydrogen recovery is used as fuel in the fired reactor, optimizing heat flux and energy usage.
Techno-Economic Assessment of Membrane-Assisted Ammonia Cracking
The techno-economic assessment of membrane-assisted ammonia cracking reveals significant potential for cost-effective hydrogen production. Initial capital expenditure analysis indicates that membrane reactor systems require 15-25% higher upfront investment compared to conventional cracking technologies, primarily due to specialized membrane materials and complex integration requirements. However, this is offset by operational cost advantages over the system lifetime.
Operational expenditure calculations demonstrate that membrane-assisted systems can achieve 20-30% reduction in energy consumption compared to traditional ammonia cracking methods. This efficiency gain stems from the selective hydrogen removal that shifts reaction equilibrium, allowing operation at lower temperatures (400-500°C versus 650-850°C for conventional systems) and reduced pressure requirements.
Sensitivity analysis reveals that membrane performance parameters, particularly hydrogen selectivity and permeance, are the most critical factors affecting economic viability. Current palladium-based membranes with selectivity >99.9% and permeance of 1.0-2.5×10^-6 mol/m²·s·Pa represent the economic sweet spot, though ceramic and metallic composite membranes show promise for cost reduction.
Levelized cost of hydrogen (LCOH) calculations indicate potential production costs of $2.50-3.20/kg H₂ using membrane-assisted ammonia cracking, compared to $3.00-4.00/kg H₂ for conventional methods. This advantage becomes more pronounced when considering carbon pricing scenarios, as membrane systems generate fewer emissions during operation.
Scale-up economics demonstrate favorable returns at industrial scales above 50 tons/day of hydrogen production, with payback periods of 4-6 years depending on regional energy prices and regulatory frameworks. Small-scale applications (<1 ton/day) remain challenging economically but show promise for distributed hydrogen networks where transportation costs are significant.
Market adoption modeling suggests that membrane-assisted ammonia cracking could capture 15-20% of the hydrogen production market by 2030, contingent upon continued improvements in membrane durability and cost reduction through manufacturing optimization. The technology appears particularly competitive in regions with established ammonia infrastructure and high natural gas prices.
Operational expenditure calculations demonstrate that membrane-assisted systems can achieve 20-30% reduction in energy consumption compared to traditional ammonia cracking methods. This efficiency gain stems from the selective hydrogen removal that shifts reaction equilibrium, allowing operation at lower temperatures (400-500°C versus 650-850°C for conventional systems) and reduced pressure requirements.
Sensitivity analysis reveals that membrane performance parameters, particularly hydrogen selectivity and permeance, are the most critical factors affecting economic viability. Current palladium-based membranes with selectivity >99.9% and permeance of 1.0-2.5×10^-6 mol/m²·s·Pa represent the economic sweet spot, though ceramic and metallic composite membranes show promise for cost reduction.
Levelized cost of hydrogen (LCOH) calculations indicate potential production costs of $2.50-3.20/kg H₂ using membrane-assisted ammonia cracking, compared to $3.00-4.00/kg H₂ for conventional methods. This advantage becomes more pronounced when considering carbon pricing scenarios, as membrane systems generate fewer emissions during operation.
Scale-up economics demonstrate favorable returns at industrial scales above 50 tons/day of hydrogen production, with payback periods of 4-6 years depending on regional energy prices and regulatory frameworks. Small-scale applications (<1 ton/day) remain challenging economically but show promise for distributed hydrogen networks where transportation costs are significant.
Market adoption modeling suggests that membrane-assisted ammonia cracking could capture 15-20% of the hydrogen production market by 2030, contingent upon continued improvements in membrane durability and cost reduction through manufacturing optimization. The technology appears particularly competitive in regions with established ammonia infrastructure and high natural gas prices.
Environmental Impact and Carbon Footprint Analysis
Ammonia cracking with hydrogen membrane reactors presents a significant opportunity for clean hydrogen production, but its environmental implications must be thoroughly assessed. The carbon footprint of this technology varies considerably depending on the ammonia production method. When using green ammonia produced from renewable electricity, the overall process can achieve near-zero carbon emissions. However, conventional ammonia production via the Haber-Bosch process using natural gas contributes approximately 1.8% of global CO2 emissions, with each ton of ammonia generating 1.6-1.8 tons of CO2.
Hydrogen membrane reactors for ammonia cracking offer substantial environmental benefits compared to traditional methods. By enabling lower operating temperatures and improving conversion efficiency, these reactors reduce energy consumption by 15-30%. This translates directly to lower greenhouse gas emissions when powered by conventional energy sources. Additionally, the selective removal of hydrogen through membranes shifts reaction equilibrium, reducing the need for excess heat and further decreasing the carbon footprint.
Life cycle assessment (LCA) studies indicate that membrane-assisted ammonia cracking can reduce overall greenhouse gas emissions by up to 40% compared to conventional steam methane reforming when considering the entire hydrogen production pathway. The environmental advantage becomes even more pronounced when renewable energy powers the cracking process, potentially achieving carbon neutrality in hydrogen production.
Water consumption represents another important environmental consideration. Membrane reactors typically require less cooling water than conventional high-temperature cracking systems, reducing water footprint by approximately 25%. This benefit is particularly valuable in water-stressed regions where hydrogen production facilities might be deployed.
The materials used in hydrogen membrane reactors also warrant environmental scrutiny. Palladium-based membranes contain precious metals with significant mining impacts, while ceramic and polymer membranes generally have lower environmental footprints. Emerging research on membrane recycling shows promise for reducing raw material demands and associated environmental impacts, with potential recovery rates exceeding 90% for precious metal components.
Local air quality benefits significantly from membrane-assisted ammonia cracking compared to fossil fuel-based hydrogen production. The process eliminates SOx and particulate emissions associated with coal gasification and reduces NOx formation due to lower operating temperatures. When replacing diesel or gasoline in transportation applications, hydrogen from this pathway can reduce particulate matter emissions by over 99% and nitrogen oxide emissions by 90-95%.
Hydrogen membrane reactors for ammonia cracking offer substantial environmental benefits compared to traditional methods. By enabling lower operating temperatures and improving conversion efficiency, these reactors reduce energy consumption by 15-30%. This translates directly to lower greenhouse gas emissions when powered by conventional energy sources. Additionally, the selective removal of hydrogen through membranes shifts reaction equilibrium, reducing the need for excess heat and further decreasing the carbon footprint.
Life cycle assessment (LCA) studies indicate that membrane-assisted ammonia cracking can reduce overall greenhouse gas emissions by up to 40% compared to conventional steam methane reforming when considering the entire hydrogen production pathway. The environmental advantage becomes even more pronounced when renewable energy powers the cracking process, potentially achieving carbon neutrality in hydrogen production.
Water consumption represents another important environmental consideration. Membrane reactors typically require less cooling water than conventional high-temperature cracking systems, reducing water footprint by approximately 25%. This benefit is particularly valuable in water-stressed regions where hydrogen production facilities might be deployed.
The materials used in hydrogen membrane reactors also warrant environmental scrutiny. Palladium-based membranes contain precious metals with significant mining impacts, while ceramic and polymer membranes generally have lower environmental footprints. Emerging research on membrane recycling shows promise for reducing raw material demands and associated environmental impacts, with potential recovery rates exceeding 90% for precious metal components.
Local air quality benefits significantly from membrane-assisted ammonia cracking compared to fossil fuel-based hydrogen production. The process eliminates SOx and particulate emissions associated with coal gasification and reduces NOx formation due to lower operating temperatures. When replacing diesel or gasoline in transportation applications, hydrogen from this pathway can reduce particulate matter emissions by over 99% and nitrogen oxide emissions by 90-95%.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!