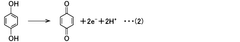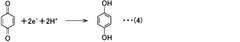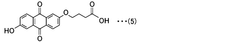Design Principles For Quinone Molecules In Aqueous Redox Flow Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quinone Molecules Background and Research Objectives
Quinone molecules have emerged as promising candidates for aqueous redox flow batteries (ARFBs) due to their unique electrochemical properties and structural versatility. The history of quinone research in energy storage can be traced back to the early 2000s, when researchers began exploring organic molecules as alternatives to metal-based electrolytes. This shift was driven by increasing concerns about the cost, environmental impact, and resource limitations of traditional metal-based systems such as vanadium redox flow batteries.
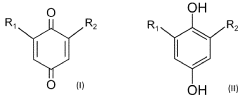
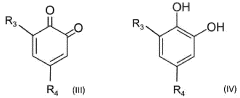
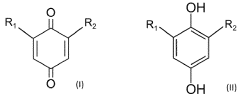
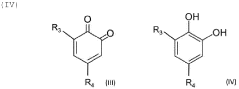
The fundamental structure of quinones consists of a cyclic conjugated system with two carbonyl groups, which can undergo reversible redox reactions by accepting and donating electrons. This electrochemical behavior makes them particularly suitable for energy storage applications. The evolution of quinone research has progressed from simple benzoquinone structures to more complex derivatives, including anthraquinones, naphthoquinones, and various functionalized variants designed to enhance specific properties.
Current technological trends in quinone development focus on addressing key challenges such as solubility in aqueous electrolytes, stability during cycling, and energy density optimization. Researchers are exploring various molecular engineering approaches, including the strategic placement of functional groups to enhance water solubility while maintaining electrochemical performance. Hydroxyl, sulfonic acid, and carboxylic acid groups have been particularly effective for improving aqueous solubility.
The primary technical objectives for quinone research in ARFBs include achieving high energy density (>25 Wh/L), extended cycle life (>1000 cycles), rapid charge-discharge capabilities, and cost-effectiveness (<$100/kWh). Additionally, researchers aim to develop quinone molecules that operate efficiently within the electrochemical stability window of water to prevent hydrogen and oxygen evolution reactions that can reduce efficiency and safety.
Another critical research goal involves understanding and mitigating degradation mechanisms that affect quinone performance over time. These include chemical decomposition pathways, side reactions with electrolyte components, and structural changes during repeated cycling. Advanced characterization techniques such as in-situ NMR, UV-vis spectroscopy, and computational modeling are being employed to gain insights into these processes.
The development of design principles for quinone molecules represents a convergence of organic chemistry, electrochemistry, and materials science. By systematically investigating structure-property relationships, researchers aim to establish predictive frameworks that can guide the rational design of next-generation quinone molecules with optimized properties for specific ARFB applications, from grid-scale energy storage to smaller distributed systems.
The fundamental structure of quinones consists of a cyclic conjugated system with two carbonyl groups, which can undergo reversible redox reactions by accepting and donating electrons. This electrochemical behavior makes them particularly suitable for energy storage applications. The evolution of quinone research has progressed from simple benzoquinone structures to more complex derivatives, including anthraquinones, naphthoquinones, and various functionalized variants designed to enhance specific properties.
Current technological trends in quinone development focus on addressing key challenges such as solubility in aqueous electrolytes, stability during cycling, and energy density optimization. Researchers are exploring various molecular engineering approaches, including the strategic placement of functional groups to enhance water solubility while maintaining electrochemical performance. Hydroxyl, sulfonic acid, and carboxylic acid groups have been particularly effective for improving aqueous solubility.
The primary technical objectives for quinone research in ARFBs include achieving high energy density (>25 Wh/L), extended cycle life (>1000 cycles), rapid charge-discharge capabilities, and cost-effectiveness (<$100/kWh). Additionally, researchers aim to develop quinone molecules that operate efficiently within the electrochemical stability window of water to prevent hydrogen and oxygen evolution reactions that can reduce efficiency and safety.
Another critical research goal involves understanding and mitigating degradation mechanisms that affect quinone performance over time. These include chemical decomposition pathways, side reactions with electrolyte components, and structural changes during repeated cycling. Advanced characterization techniques such as in-situ NMR, UV-vis spectroscopy, and computational modeling are being employed to gain insights into these processes.
The development of design principles for quinone molecules represents a convergence of organic chemistry, electrochemistry, and materials science. By systematically investigating structure-property relationships, researchers aim to establish predictive frameworks that can guide the rational design of next-generation quinone molecules with optimized properties for specific ARFB applications, from grid-scale energy storage to smaller distributed systems.
Market Analysis for Aqueous Redox Flow Battery Technologies
The global market for aqueous redox flow batteries (ARFBs) is experiencing significant growth, driven by increasing demand for grid-scale energy storage solutions. As renewable energy integration accelerates worldwide, the need for efficient, long-duration storage technologies has become critical. The market value for flow batteries reached approximately $290 million in 2022 and is projected to grow at a CAGR of 15-20% through 2030, potentially reaching $1.1 billion by the end of the decade.
Quinone-based ARFBs represent an emerging segment within this market, attracting attention due to their potential cost advantages over vanadium-based systems, which currently dominate the commercial landscape. The high cost of vanadium (ranging from $20-30/kg) has created a market opportunity for organic alternatives like quinones, which can be synthesized from abundant carbon sources at potentially lower costs.
Regional analysis shows Asia-Pacific leading the ARFB market, with China accounting for over 40% of global installations. North America and Europe follow, with growing investments in grid modernization and renewable integration driving adoption. Japan and South Korea have also emerged as significant markets due to their limited land availability and energy security concerns.
By application segment, utility-scale storage represents the largest market share (approximately 65%), followed by industrial applications (20%) and microgrids (15%). The increasing penetration of intermittent renewable energy sources, particularly solar and wind, is the primary driver for utility-scale deployment.
Key customer segments include electric utilities, renewable energy developers, and industrial facilities seeking to manage peak demand charges. These customers prioritize levelized cost of storage (LCOS), cycle life, and safety—areas where quinone-based systems must demonstrate competitive advantages to gain market share.
Market barriers for quinone-based ARFBs include competition from lithium-ion batteries, which benefit from manufacturing scale and declining costs (currently $200-300/kWh for grid applications), as well as from established flow battery chemistries. Technical challenges related to quinone stability, energy density, and membrane performance must be addressed to improve market competitiveness.
Regulatory factors are increasingly favorable for long-duration storage technologies like ARFBs. Several jurisdictions have implemented storage mandates and incentives, while carbon pricing mechanisms in Europe and parts of North America further enhance the economic case for storage paired with renewables.
The competitive landscape includes established flow battery manufacturers exploring quinone chemistry, specialized startups focused exclusively on organic flow batteries, and research institutions commercializing novel quinone molecules. Strategic partnerships between material developers and system integrators are becoming increasingly common to accelerate commercialization.
Quinone-based ARFBs represent an emerging segment within this market, attracting attention due to their potential cost advantages over vanadium-based systems, which currently dominate the commercial landscape. The high cost of vanadium (ranging from $20-30/kg) has created a market opportunity for organic alternatives like quinones, which can be synthesized from abundant carbon sources at potentially lower costs.
Regional analysis shows Asia-Pacific leading the ARFB market, with China accounting for over 40% of global installations. North America and Europe follow, with growing investments in grid modernization and renewable integration driving adoption. Japan and South Korea have also emerged as significant markets due to their limited land availability and energy security concerns.
By application segment, utility-scale storage represents the largest market share (approximately 65%), followed by industrial applications (20%) and microgrids (15%). The increasing penetration of intermittent renewable energy sources, particularly solar and wind, is the primary driver for utility-scale deployment.
Key customer segments include electric utilities, renewable energy developers, and industrial facilities seeking to manage peak demand charges. These customers prioritize levelized cost of storage (LCOS), cycle life, and safety—areas where quinone-based systems must demonstrate competitive advantages to gain market share.
Market barriers for quinone-based ARFBs include competition from lithium-ion batteries, which benefit from manufacturing scale and declining costs (currently $200-300/kWh for grid applications), as well as from established flow battery chemistries. Technical challenges related to quinone stability, energy density, and membrane performance must be addressed to improve market competitiveness.
Regulatory factors are increasingly favorable for long-duration storage technologies like ARFBs. Several jurisdictions have implemented storage mandates and incentives, while carbon pricing mechanisms in Europe and parts of North America further enhance the economic case for storage paired with renewables.
The competitive landscape includes established flow battery manufacturers exploring quinone chemistry, specialized startups focused exclusively on organic flow batteries, and research institutions commercializing novel quinone molecules. Strategic partnerships between material developers and system integrators are becoming increasingly common to accelerate commercialization.
Current Challenges in Quinone-Based Flow Battery Development
Despite the promising attributes of quinone molecules for aqueous redox flow batteries (ARFBs), several significant challenges impede their widespread commercial implementation. The primary obstacle remains the limited stability of quinone compounds in aqueous environments. Many quinone derivatives undergo side reactions, particularly Michael addition and nucleophilic addition, leading to capacity fade during cycling. This chemical degradation is especially pronounced at extreme pH values, creating a narrow operational window for long-term stability.
Solubility limitations represent another critical challenge. While high solubility is essential for achieving competitive energy densities, many quinone molecules exhibit insufficient solubility in aqueous electrolytes. Current research indicates that most quinone compounds struggle to exceed 1-2 M concentration, falling short of the theoretical energy density requirements for commercial viability. Structural modifications to enhance solubility often compromise redox potential or stability, creating a complex optimization problem.
Crossover of active materials through the membrane constitutes a persistent issue in quinone-based systems. The relatively small molecular size of many quinone derivatives enables their migration across ion-exchange membranes, resulting in capacity loss and decreased coulombic efficiency. This phenomenon necessitates frequent electrolyte rebalancing, increasing operational complexity and maintenance costs.
The redox potential range of quinone molecules presents another significant limitation. While anthraquinones offer suitable negative electrolyte candidates, finding complementary positive electrolyte quinone compounds with appropriate potentials remains challenging. The voltage gap between positive and negative electrolytes directly impacts energy density, with current quinone-based systems typically limited to cell voltages below 1.2 V.
Manufacturing scalability and cost considerations further complicate quinone implementation. Many high-performance quinone derivatives require multi-step synthesis routes with expensive reagents and purification processes. The trade-off between performance and production cost creates barriers to industrial-scale adoption, particularly when competing with established vanadium-based systems.
Lastly, the environmental impact of quinone-based electrolytes requires careful assessment. While organic electrolytes offer potential advantages over metal-based alternatives, comprehensive life cycle analyses are needed to evaluate their true sustainability. Questions regarding biodegradability, toxicity, and end-of-life management remain insufficiently addressed in current research literature.
Solubility limitations represent another critical challenge. While high solubility is essential for achieving competitive energy densities, many quinone molecules exhibit insufficient solubility in aqueous electrolytes. Current research indicates that most quinone compounds struggle to exceed 1-2 M concentration, falling short of the theoretical energy density requirements for commercial viability. Structural modifications to enhance solubility often compromise redox potential or stability, creating a complex optimization problem.
Crossover of active materials through the membrane constitutes a persistent issue in quinone-based systems. The relatively small molecular size of many quinone derivatives enables their migration across ion-exchange membranes, resulting in capacity loss and decreased coulombic efficiency. This phenomenon necessitates frequent electrolyte rebalancing, increasing operational complexity and maintenance costs.
The redox potential range of quinone molecules presents another significant limitation. While anthraquinones offer suitable negative electrolyte candidates, finding complementary positive electrolyte quinone compounds with appropriate potentials remains challenging. The voltage gap between positive and negative electrolytes directly impacts energy density, with current quinone-based systems typically limited to cell voltages below 1.2 V.
Manufacturing scalability and cost considerations further complicate quinone implementation. Many high-performance quinone derivatives require multi-step synthesis routes with expensive reagents and purification processes. The trade-off between performance and production cost creates barriers to industrial-scale adoption, particularly when competing with established vanadium-based systems.
Lastly, the environmental impact of quinone-based electrolytes requires careful assessment. While organic electrolytes offer potential advantages over metal-based alternatives, comprehensive life cycle analyses are needed to evaluate their true sustainability. Questions regarding biodegradability, toxicity, and end-of-life management remain insufficiently addressed in current research literature.
Existing Quinone Design Strategies for Aqueous Environments
01 Synthesis and preparation of quinone derivatives
Various methods for synthesizing quinone molecules and their derivatives have been developed. These processes involve chemical reactions such as oxidation, reduction, and substitution to create different quinone structures with specific properties. The synthesis methods can be optimized for industrial scale production, allowing for the creation of quinones with applications in pharmaceuticals, dyes, and other chemical industries.- Synthesis and preparation methods of quinone compounds: Various methods for synthesizing quinone molecules have been developed, including oxidation of aromatic compounds, enzymatic processes, and chemical transformations. These methods allow for the production of different types of quinone derivatives with specific structural features. The synthesis approaches can be tailored to yield quinones with desired functional groups and properties for various applications in pharmaceuticals, materials science, and other fields.
- Quinones as active pharmaceutical ingredients: Quinone molecules exhibit significant pharmacological activities and are used as active ingredients in pharmaceutical formulations. They demonstrate antimicrobial, anticancer, and anti-inflammatory properties. The unique redox properties of quinones enable them to interact with biological systems, making them valuable therapeutic agents. Research focuses on developing quinone-based drugs with enhanced efficacy and reduced side effects for treating various diseases.
- Quinones in electronic and energy storage applications: Quinone compounds are utilized in electronic devices and energy storage systems due to their redox properties. They serve as electron transfer mediators in batteries, supercapacitors, and other electrochemical systems. The ability of quinones to undergo reversible reduction and oxidation makes them valuable components in sustainable energy technologies. Research in this area focuses on enhancing the stability and performance of quinone-based materials for improved energy storage solutions.
- Polymer applications of quinone derivatives: Quinone molecules are incorporated into polymer structures to impart specific properties such as color, conductivity, or reactivity. They can function as crosslinking agents, polymerization initiators, or functional components within polymer chains. The integration of quinones into polymeric materials enables the development of advanced materials with tailored characteristics for applications in coatings, adhesives, and specialty polymers with enhanced performance properties.
- Environmental and biological roles of quinones: Quinones play important roles in biological systems and environmental processes. They function as electron carriers in cellular respiration and photosynthesis. In environmental applications, quinones are studied for their potential in bioremediation and as indicators of ecological health. Research explores the natural occurrence of quinones in various organisms and their functions in biochemical pathways, as well as their applications in environmental monitoring and remediation technologies.
02 Quinones in pharmaceutical applications
Quinone molecules exhibit significant biological activities that make them valuable in pharmaceutical applications. They can function as active ingredients in medications targeting various diseases, including cancer, infections, and inflammatory conditions. The unique redox properties of quinones allow them to interact with biological systems in ways that can be harnessed for therapeutic purposes, such as disrupting cellular processes in cancer cells or combating pathogenic microorganisms.Expand Specific Solutions03 Quinones in electronic and energy storage applications
Quinone molecules possess electrochemical properties that make them suitable for applications in electronic devices and energy storage systems. They can undergo reversible redox reactions, making them valuable components in batteries, capacitors, and other energy storage technologies. Additionally, quinones can be incorporated into electronic materials to enhance conductivity or create specific electronic properties in devices such as organic semiconductors and sensors.Expand Specific Solutions04 Quinones as industrial chemical intermediates
Quinone molecules serve as important intermediates in various industrial chemical processes. They are used in the production of dyes, pigments, and other specialty chemicals. The reactive nature of quinones makes them valuable building blocks for creating complex chemical structures. Industrial applications leverage the ability of quinones to participate in addition reactions, substitutions, and polymerization processes to create commercially important products.Expand Specific Solutions05 Quinones in biological and environmental systems
Quinones play significant roles in biological and environmental systems. In biology, they function as electron carriers in processes like photosynthesis and cellular respiration. Environmental applications include their use in bioremediation processes, where quinones can facilitate the breakdown of pollutants. Research has focused on understanding how quinones interact with biological molecules and environmental contaminants, leading to applications in environmental monitoring and remediation technologies.Expand Specific Solutions
Leading Organizations in Redox Flow Battery Research
The aqueous redox flow battery (ARFB) market utilizing quinone molecules is in an early growth phase, characterized by intensive research and development activities. Academic institutions like Harvard College, University of Southern California, and Nanjing University are leading fundamental research, while companies such as CMBlu Energy AG, KEMIWATT, and Standard Energy are working to commercialize these technologies. The market is projected to expand significantly as renewable energy storage demands increase, though technical challenges remain. Current technology maturity varies, with research institutions focusing on molecular design optimization while companies like Mitsubishi Heavy Industries and Lockheed Martin Advanced Energy Storage are developing scalable systems. Collaboration between academia and industry is accelerating progress toward commercially viable quinone-based ARFBs with improved energy density and cycle life.
President & Fellows of Harvard College
Technical Solution: Harvard College has pioneered innovative design principles for quinone molecules in aqueous redox flow batteries (ARFBs). Their approach focuses on molecular engineering of organic compounds, particularly quinones, to optimize electrochemical properties. They've developed anthraquinone-based compounds with sulfonic acid groups that enhance water solubility while maintaining electrochemical reversibility. Their research demonstrated that 9,10-anthraquinone-2,7-disulfonic acid (AQDS) can achieve high solubility (>1M) in aqueous solutions and fast kinetics with carbon electrodes. Harvard researchers have also explored strategies to tune redox potentials through systematic modification of electron-donating and withdrawing groups on the quinone core structure, enabling precise control over cell voltage. Additionally, they've investigated the relationship between molecular structure and cycling stability, identifying mechanisms to mitigate capacity fade through strategic molecular design that prevents side reactions and decomposition pathways.
Strengths: Superior molecular engineering expertise allowing precise tuning of electrochemical properties; extensive research on structure-property relationships enabling rational design of high-performance quinones. Weaknesses: Some of their quinone derivatives may face challenges in long-term stability under actual operating conditions; potential high synthesis costs for complex molecular structures could limit commercial viability.
CMBlu Energy AG
Technical Solution: CMBlu Energy has developed a proprietary organic redox flow battery technology centered on quinone-based molecules specifically engineered for grid-scale energy storage applications. Their design principles focus on utilizing modified anthraquinone derivatives with optimized solubility characteristics in aqueous electrolytes. CMBlu's approach involves systematic molecular engineering to enhance electron transfer kinetics while maintaining long-term cycling stability. Their technology employs functionalized quinones with carefully positioned substituent groups that increase solubility beyond 2M concentration while preserving fast redox kinetics. The company has pioneered methods to mitigate capacity fade by designing molecular structures resistant to side reactions and chemical degradation during extended cycling. CMBlu's electrolyte formulations incorporate specific additives that work synergistically with their quinone molecules to enhance performance across wide temperature ranges. Their system architecture integrates advanced membrane technology specifically matched to their quinone chemistry, minimizing crossover effects and extending operational lifetime.
Strengths: Highly optimized quinone molecules specifically designed for commercial viability; integrated system approach that addresses both chemistry and engineering challenges simultaneously. Weaknesses: Proprietary nature of their specific molecular designs limits academic validation; potential challenges in scaling production of specialized quinone derivatives to industrial quantities at competitive costs.
Key Molecular Structure-Property Relationships in Quinones
Stable aqueous compositions comprising quinones, and use thereof in redox flow batteries
PatentWO2021105322A1
Innovation
- An aqueous composition comprising quinones stabilized by phosphate ions and/or hydrogen phosphate ions, allowing for stable aqueous solutions that can be used in redox flow batteries without additional expensive measures, and enabling photoreduction reactions in an aqueous medium.
Redox flow battery
PatentWO2023120449A1
Innovation
- A redox flow battery design with separate chambers and electrolyte tanks, using quinones as active material in the second electrolyte, includes a control device that stops charging when a preset upper limit of charging rate is reached to prevent decomposition, and maintains optimal pH and temperature ranges to enhance operational efficiency.
Environmental Impact and Sustainability of Quinone-Based Systems
The environmental impact of quinone-based aqueous redox flow batteries (ARFBs) represents a critical consideration in their development and deployment. Quinone molecules, derived from abundant natural sources such as lignin and other plant materials, offer significant sustainability advantages over traditional metal-based flow battery systems. Their organic nature provides inherent biodegradability potential, reducing end-of-life environmental concerns that plague metal-based alternatives.
Life cycle assessments of quinone-based ARFBs reveal substantially lower carbon footprints compared to vanadium flow batteries, primarily due to reduced energy requirements during material extraction and processing. The elimination of rare earth metals and toxic heavy metals significantly decreases environmental risks associated with mining operations, including habitat destruction, water pollution, and soil contamination. Furthermore, the water-based electrolyte systems eliminate the need for volatile organic solvents, reducing both environmental hazards and workplace safety concerns.
Manufacturing processes for quinone molecules can be optimized for green chemistry principles, utilizing bio-derived precursors and environmentally benign reaction pathways. Recent advances in synthetic methodologies have demonstrated potential for near-zero waste production systems, particularly when employing enzymatic or microbial synthesis routes. These approaches align with circular economy principles, where waste streams from other industries can serve as feedstock for quinone production.
The operational phase of quinone-based ARFBs demonstrates minimal environmental impact, with negligible emissions and low maintenance requirements. The absence of membrane degradation issues common in acidic systems extends component lifetimes, reducing replacement frequency and associated material consumption. Water management remains a challenge, though closed-loop systems have demonstrated over 98% water recovery rates in pilot installations.
End-of-life considerations for quinone-based systems show promising recyclability pathways. The organic nature of the active materials allows for potential biodegradation under controlled conditions or recovery through separation techniques for reuse. Research indicates that up to 85% of quinone compounds can be reclaimed from spent electrolytes using advanced separation methods, significantly reducing waste generation compared to conventional battery technologies.
Regulatory frameworks increasingly favor these environmentally advantageous systems, with several jurisdictions implementing incentives for sustainable energy storage solutions. The reduced toxicity profile of quinone-based systems simplifies compliance with hazardous material regulations, potentially accelerating commercial adoption and deployment in environmentally sensitive areas where conventional battery technologies face restrictions.
Life cycle assessments of quinone-based ARFBs reveal substantially lower carbon footprints compared to vanadium flow batteries, primarily due to reduced energy requirements during material extraction and processing. The elimination of rare earth metals and toxic heavy metals significantly decreases environmental risks associated with mining operations, including habitat destruction, water pollution, and soil contamination. Furthermore, the water-based electrolyte systems eliminate the need for volatile organic solvents, reducing both environmental hazards and workplace safety concerns.
Manufacturing processes for quinone molecules can be optimized for green chemistry principles, utilizing bio-derived precursors and environmentally benign reaction pathways. Recent advances in synthetic methodologies have demonstrated potential for near-zero waste production systems, particularly when employing enzymatic or microbial synthesis routes. These approaches align with circular economy principles, where waste streams from other industries can serve as feedstock for quinone production.
The operational phase of quinone-based ARFBs demonstrates minimal environmental impact, with negligible emissions and low maintenance requirements. The absence of membrane degradation issues common in acidic systems extends component lifetimes, reducing replacement frequency and associated material consumption. Water management remains a challenge, though closed-loop systems have demonstrated over 98% water recovery rates in pilot installations.
End-of-life considerations for quinone-based systems show promising recyclability pathways. The organic nature of the active materials allows for potential biodegradation under controlled conditions or recovery through separation techniques for reuse. Research indicates that up to 85% of quinone compounds can be reclaimed from spent electrolytes using advanced separation methods, significantly reducing waste generation compared to conventional battery technologies.
Regulatory frameworks increasingly favor these environmentally advantageous systems, with several jurisdictions implementing incentives for sustainable energy storage solutions. The reduced toxicity profile of quinone-based systems simplifies compliance with hazardous material regulations, potentially accelerating commercial adoption and deployment in environmentally sensitive areas where conventional battery technologies face restrictions.
Scalability and Commercial Viability Assessment
The scalability of quinone-based aqueous redox flow batteries (ARFBs) represents a critical factor in their transition from laboratory research to commercial deployment. Current manufacturing processes for quinone molecules demonstrate promising potential for large-scale production, with established synthetic routes that can be adapted from existing chemical industry infrastructure. The relatively simple molecular structure of many quinone derivatives enables cost-effective synthesis compared to more complex organometallic alternatives.
Cost analysis indicates that quinone precursors derived from abundant natural sources such as lignin or petroleum byproducts could significantly reduce production expenses. Preliminary economic assessments suggest that at scale, quinone-based electrolytes could achieve costs below $100/kWh, approaching the Department of Energy's target for grid-scale energy storage systems. This economic viability is further enhanced by the absence of precious metals in these systems.
Supply chain considerations for quinone-based ARFBs appear favorable, with raw materials widely available and not subject to geopolitical constraints that affect certain metal-based battery technologies. The aqueous nature of these systems also reduces safety concerns and associated regulatory compliance costs that impact commercial viability of other battery technologies.
Manufacturing scalability assessment reveals that quinone production can leverage existing chemical processing facilities with moderate modifications. The liquid nature of flow battery components simplifies assembly compared to solid-state batteries, potentially reducing manufacturing complexity and capital expenditure requirements for production facilities.
Market adoption barriers primarily center on the limited energy density of current quinone-based systems, which restricts their application to stationary storage rather than transportation. However, this limitation is less significant for grid-scale applications where space constraints are less critical than cost and cycle life. The demonstrated long-term stability of newer quinone derivatives addresses previous concerns about electrolyte degradation that had threatened commercial viability.
Regulatory pathway analysis suggests favorable conditions for quinone-based ARFBs, with their environmentally benign components likely to face fewer restrictions than systems containing toxic or flammable materials. This regulatory advantage could accelerate market entry timelines and reduce compliance costs.
Investment trends indicate growing interest in quinone-based flow battery technologies, with several startups securing significant funding rounds in recent years. Strategic partnerships between academic institutions, where much of the fundamental research originates, and industrial entities positioned to scale production, are emerging as a viable commercialization pathway.
Cost analysis indicates that quinone precursors derived from abundant natural sources such as lignin or petroleum byproducts could significantly reduce production expenses. Preliminary economic assessments suggest that at scale, quinone-based electrolytes could achieve costs below $100/kWh, approaching the Department of Energy's target for grid-scale energy storage systems. This economic viability is further enhanced by the absence of precious metals in these systems.
Supply chain considerations for quinone-based ARFBs appear favorable, with raw materials widely available and not subject to geopolitical constraints that affect certain metal-based battery technologies. The aqueous nature of these systems also reduces safety concerns and associated regulatory compliance costs that impact commercial viability of other battery technologies.
Manufacturing scalability assessment reveals that quinone production can leverage existing chemical processing facilities with moderate modifications. The liquid nature of flow battery components simplifies assembly compared to solid-state batteries, potentially reducing manufacturing complexity and capital expenditure requirements for production facilities.
Market adoption barriers primarily center on the limited energy density of current quinone-based systems, which restricts their application to stationary storage rather than transportation. However, this limitation is less significant for grid-scale applications where space constraints are less critical than cost and cycle life. The demonstrated long-term stability of newer quinone derivatives addresses previous concerns about electrolyte degradation that had threatened commercial viability.
Regulatory pathway analysis suggests favorable conditions for quinone-based ARFBs, with their environmentally benign components likely to face fewer restrictions than systems containing toxic or flammable materials. This regulatory advantage could accelerate market entry timelines and reduce compliance costs.
Investment trends indicate growing interest in quinone-based flow battery technologies, with several startups securing significant funding rounds in recent years. Strategic partnerships between academic institutions, where much of the fundamental research originates, and industrial entities positioned to scale production, are emerging as a viable commercialization pathway.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!