Membrane Selection And Fouling Control In Aqueous ORFBs
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ORFB Membrane Technology Background and Objectives
Organic Redox Flow Batteries (ORFBs) have emerged as a promising energy storage technology due to their scalability, flexibility, and potential for cost-effectiveness. The development of ORFBs traces back to the early 2000s when researchers began exploring organic molecules as alternatives to metal-based electrolytes in conventional redox flow batteries. This shift was driven by concerns over resource scarcity, environmental impact, and cost limitations associated with vanadium and other metal-based systems.
The evolution of ORFB technology has been characterized by significant advancements in organic electroactive materials, electrolyte formulations, and system design. Early iterations faced challenges related to energy density, cycle life, and stability. However, the past decade has witnessed remarkable progress in addressing these limitations through molecular engineering and system optimization.
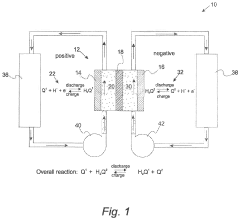
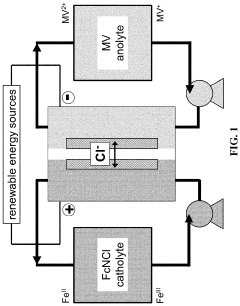
Membrane technology represents a critical component in ORFB development, serving as the physical separator between positive and negative electrolytes while facilitating selective ion transport. Initially, ORFB systems adopted membranes designed for other applications, particularly from fuel cell technologies. This approach, while expedient, failed to address the unique requirements of organic electrolyte systems.
The technical objectives for membrane development in aqueous ORFBs center on several key parameters: ionic conductivity, selectivity, chemical stability, mechanical durability, and cost-effectiveness. Achieving optimal performance requires balancing these often competing factors. Specifically, membranes must exhibit high ionic conductivity to minimize resistance losses while maintaining excellent selectivity to prevent crossover of active organic species.
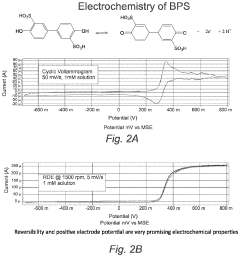
Membrane fouling presents a particularly challenging obstacle in aqueous ORFB systems. Unlike their metal-based counterparts, organic electrolytes can interact with membrane materials through various mechanisms including adsorption, precipitation, and chemical degradation. These interactions lead to progressive performance deterioration through increased resistance, reduced selectivity, and compromised mechanical integrity.
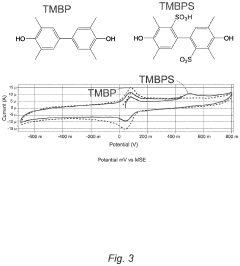
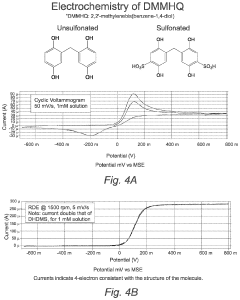
Current research trends focus on developing specialized membrane materials tailored specifically for aqueous ORFB environments. These include modified perfluorinated membranes, hydrocarbon-based alternatives, composite structures, and novel polymer architectures. Additionally, surface modification techniques and anti-fouling strategies are being explored to enhance long-term operational stability.
The technological trajectory suggests a move toward integrated membrane design approaches that consider the entire battery system rather than treating the membrane as an isolated component. This holistic perspective acknowledges the complex interplay between membrane properties, electrolyte characteristics, and operating conditions in determining overall system performance and longevity.
The evolution of ORFB technology has been characterized by significant advancements in organic electroactive materials, electrolyte formulations, and system design. Early iterations faced challenges related to energy density, cycle life, and stability. However, the past decade has witnessed remarkable progress in addressing these limitations through molecular engineering and system optimization.
Membrane technology represents a critical component in ORFB development, serving as the physical separator between positive and negative electrolytes while facilitating selective ion transport. Initially, ORFB systems adopted membranes designed for other applications, particularly from fuel cell technologies. This approach, while expedient, failed to address the unique requirements of organic electrolyte systems.
The technical objectives for membrane development in aqueous ORFBs center on several key parameters: ionic conductivity, selectivity, chemical stability, mechanical durability, and cost-effectiveness. Achieving optimal performance requires balancing these often competing factors. Specifically, membranes must exhibit high ionic conductivity to minimize resistance losses while maintaining excellent selectivity to prevent crossover of active organic species.
Membrane fouling presents a particularly challenging obstacle in aqueous ORFB systems. Unlike their metal-based counterparts, organic electrolytes can interact with membrane materials through various mechanisms including adsorption, precipitation, and chemical degradation. These interactions lead to progressive performance deterioration through increased resistance, reduced selectivity, and compromised mechanical integrity.
Current research trends focus on developing specialized membrane materials tailored specifically for aqueous ORFB environments. These include modified perfluorinated membranes, hydrocarbon-based alternatives, composite structures, and novel polymer architectures. Additionally, surface modification techniques and anti-fouling strategies are being explored to enhance long-term operational stability.
The technological trajectory suggests a move toward integrated membrane design approaches that consider the entire battery system rather than treating the membrane as an isolated component. This holistic perspective acknowledges the complex interplay between membrane properties, electrolyte characteristics, and operating conditions in determining overall system performance and longevity.
Market Analysis for ORFB Energy Storage Solutions
The global energy storage market is experiencing significant growth, with the Organic Redox Flow Battery (ORFB) segment emerging as a promising technology for grid-scale applications. Current market valuations place the overall flow battery market at approximately $290 million in 2023, with projections indicating growth to reach $1.1 billion by 2030, representing a compound annual growth rate (CAGR) of 21.3%. Within this broader market, aqueous ORFBs are gaining traction due to their potential cost advantages and environmental benefits compared to traditional vanadium flow batteries.
Market demand for ORFB technology is primarily driven by the increasing integration of renewable energy sources into power grids worldwide. The intermittent nature of solar and wind power generation necessitates efficient, large-scale energy storage solutions. ORFBs, with their scalable capacity, long cycle life, and decoupled power and energy characteristics, are particularly well-positioned to address this need.
Regional analysis reveals varying adoption rates and market potential. North America currently leads in ORFB research and deployment, with significant investments from both government agencies and private sector entities. The European market shows strong growth potential, supported by aggressive renewable energy targets and favorable regulatory frameworks. The Asia-Pacific region, particularly China, Japan, and South Korea, is rapidly expanding its ORFB market presence through substantial research funding and demonstration projects.
Customer segmentation indicates three primary market sectors for ORFB technology: utility-scale energy storage for grid stabilization, commercial and industrial applications for peak shaving and backup power, and remote or off-grid installations requiring reliable energy storage solutions. The utility sector represents the largest market share, accounting for approximately 65% of current deployments.
Competitive analysis reveals that while traditional lithium-ion batteries currently dominate the energy storage market, ORFBs offer distinct advantages for long-duration storage applications (4+ hours). The membrane selection and fouling control challenges addressed in this research directly impact ORFB cost structures and performance metrics, with membrane components typically representing 15-20% of total system costs.
Market barriers include high initial capital costs, limited commercial-scale demonstrations, and technical challenges related to energy density and system efficiency. However, recent advancements in membrane technology and fouling mitigation strategies are progressively addressing these limitations, potentially accelerating market adoption rates.
Forecast models suggest that successful resolution of membrane fouling issues could reduce ORFB system costs by 25-30%, potentially accelerating market penetration and expanding addressable markets to include medium-duration storage applications currently dominated by lithium-ion technologies.
Market demand for ORFB technology is primarily driven by the increasing integration of renewable energy sources into power grids worldwide. The intermittent nature of solar and wind power generation necessitates efficient, large-scale energy storage solutions. ORFBs, with their scalable capacity, long cycle life, and decoupled power and energy characteristics, are particularly well-positioned to address this need.
Regional analysis reveals varying adoption rates and market potential. North America currently leads in ORFB research and deployment, with significant investments from both government agencies and private sector entities. The European market shows strong growth potential, supported by aggressive renewable energy targets and favorable regulatory frameworks. The Asia-Pacific region, particularly China, Japan, and South Korea, is rapidly expanding its ORFB market presence through substantial research funding and demonstration projects.
Customer segmentation indicates three primary market sectors for ORFB technology: utility-scale energy storage for grid stabilization, commercial and industrial applications for peak shaving and backup power, and remote or off-grid installations requiring reliable energy storage solutions. The utility sector represents the largest market share, accounting for approximately 65% of current deployments.
Competitive analysis reveals that while traditional lithium-ion batteries currently dominate the energy storage market, ORFBs offer distinct advantages for long-duration storage applications (4+ hours). The membrane selection and fouling control challenges addressed in this research directly impact ORFB cost structures and performance metrics, with membrane components typically representing 15-20% of total system costs.
Market barriers include high initial capital costs, limited commercial-scale demonstrations, and technical challenges related to energy density and system efficiency. However, recent advancements in membrane technology and fouling mitigation strategies are progressively addressing these limitations, potentially accelerating market adoption rates.
Forecast models suggest that successful resolution of membrane fouling issues could reduce ORFB system costs by 25-30%, potentially accelerating market penetration and expanding addressable markets to include medium-duration storage applications currently dominated by lithium-ion technologies.
Current Membrane Challenges in Aqueous ORFBs
Aqueous organic redox flow batteries (ORFBs) represent a promising energy storage technology due to their scalability, safety, and potential cost-effectiveness. However, membrane-related challenges significantly hinder their widespread commercial adoption. The ion-exchange membrane (IEM) serves as a critical component in ORFBs, responsible for preventing active material crossover while facilitating ion transport to maintain charge balance.
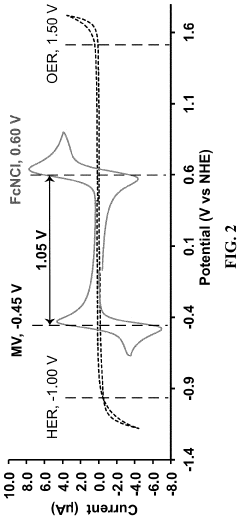
Current commercial membranes, primarily designed for fuel cells or other electrochemical applications, exhibit suboptimal performance in ORFB environments. Nafion and other perfluorinated membranes demonstrate excellent ionic conductivity but suffer from high permeability to organic active materials, resulting in capacity fade and efficiency losses. This crossover phenomenon remains one of the most persistent challenges in ORFB development.
Hydrocarbon-based membranes offer improved selectivity against organic molecules but typically exhibit lower ionic conductivity, creating an inherent performance trade-off. This conductivity-selectivity dilemma represents a fundamental challenge that membrane developers must address to advance ORFB technology.
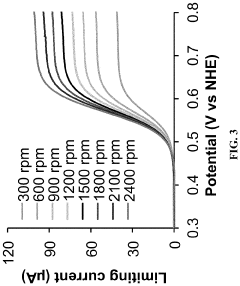
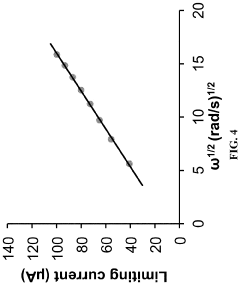
Membrane fouling presents another significant obstacle in aqueous ORFBs. During extended operation, active organic materials and their degradation products can adsorb onto and within membrane structures, progressively reducing ionic conductivity and increasing area-specific resistance. This fouling mechanism differs from traditional water treatment fouling and requires specialized mitigation strategies.
Chemical stability issues further complicate membrane selection, as membranes must withstand both oxidative and reductive environments simultaneously while exposed to reactive organic species. Many commercial membranes experience accelerated degradation under these conditions, leading to diminished mechanical properties and shortened operational lifetimes.
Cost considerations remain paramount, with high-performance membranes like Nafion contributing significantly to overall system expenses. The current price point of approximately $500/m² for perfluorinated membranes creates economic barriers to large-scale ORFB deployment, necessitating more cost-effective alternatives.
Thickness optimization presents another technical challenge, as thinner membranes reduce ionic resistance but increase crossover rates. Finding the optimal thickness that balances these competing factors requires sophisticated modeling and experimental validation specific to each ORFB chemistry.
Scaling and manufacturing considerations introduce additional complexities, as laboratory-scale membrane developments often face significant hurdles in translation to industrial production. Ensuring consistent quality, mechanical durability, and dimensional stability at larger scales remains problematic for many promising membrane materials.
Current commercial membranes, primarily designed for fuel cells or other electrochemical applications, exhibit suboptimal performance in ORFB environments. Nafion and other perfluorinated membranes demonstrate excellent ionic conductivity but suffer from high permeability to organic active materials, resulting in capacity fade and efficiency losses. This crossover phenomenon remains one of the most persistent challenges in ORFB development.
Hydrocarbon-based membranes offer improved selectivity against organic molecules but typically exhibit lower ionic conductivity, creating an inherent performance trade-off. This conductivity-selectivity dilemma represents a fundamental challenge that membrane developers must address to advance ORFB technology.
Membrane fouling presents another significant obstacle in aqueous ORFBs. During extended operation, active organic materials and their degradation products can adsorb onto and within membrane structures, progressively reducing ionic conductivity and increasing area-specific resistance. This fouling mechanism differs from traditional water treatment fouling and requires specialized mitigation strategies.
Chemical stability issues further complicate membrane selection, as membranes must withstand both oxidative and reductive environments simultaneously while exposed to reactive organic species. Many commercial membranes experience accelerated degradation under these conditions, leading to diminished mechanical properties and shortened operational lifetimes.
Cost considerations remain paramount, with high-performance membranes like Nafion contributing significantly to overall system expenses. The current price point of approximately $500/m² for perfluorinated membranes creates economic barriers to large-scale ORFB deployment, necessitating more cost-effective alternatives.
Thickness optimization presents another technical challenge, as thinner membranes reduce ionic resistance but increase crossover rates. Finding the optimal thickness that balances these competing factors requires sophisticated modeling and experimental validation specific to each ORFB chemistry.
Scaling and manufacturing considerations introduce additional complexities, as laboratory-scale membrane developments often face significant hurdles in translation to industrial production. Ensuring consistent quality, mechanical durability, and dimensional stability at larger scales remains problematic for many promising membrane materials.
Current Membrane Selection Strategies and Fouling Control Methods
01 Surface modification of membranes to prevent fouling
Surface modification techniques can be applied to membranes in aqueous organic redox flow batteries to prevent fouling. These modifications include coating the membrane surface with hydrophilic polymers, applying anti-fouling agents, or introducing functional groups that repel organic contaminants. Such treatments create a protective layer that minimizes the adhesion of organic molecules to the membrane surface, thereby maintaining ion conductivity and extending membrane lifetime.- Surface modification techniques for membrane fouling control: Various surface modification techniques can be applied to membranes in ORFBs to reduce fouling. These include coating membranes with hydrophilic polymers, applying anti-fouling layers, and surface functionalization with specific chemical groups that repel organic contaminants. These modifications create a protective barrier that prevents organic molecules from adhering to the membrane surface, thereby extending membrane lifetime and maintaining battery efficiency.
- Membrane cleaning protocols and regeneration methods: Specific cleaning protocols and regeneration methods have been developed to address membrane fouling in ORFBs. These include periodic flushing with cleaning solutions, backwashing techniques, and chemical treatments that can dissolve and remove organic deposits without damaging the membrane structure. Implementing regular maintenance procedures helps restore membrane performance and extends operational lifetime of the battery system.
- Advanced membrane materials resistant to organic fouling: Novel membrane materials have been engineered specifically for resistance to organic fouling in ORFBs. These include composite membranes with specialized polymer blends, nanostructured materials with controlled pore sizes, and chemically stable materials that resist degradation from organic electrolytes. These advanced materials maintain ion selectivity while minimizing interactions with organic redox species, resulting in reduced fouling and improved long-term performance.
- Electrolyte additives and formulations to reduce membrane fouling: Specific additives and electrolyte formulations have been developed to minimize membrane fouling in ORFBs. These include stabilizing agents that prevent decomposition of organic redox species, chelating compounds that bind potential foulants, and surfactants that modify the interaction between organic molecules and membrane surfaces. Optimized electrolyte chemistry helps maintain membrane performance by reducing the formation of fouling deposits.
- Flow field and system design for fouling mitigation: Innovative flow field designs and system configurations can significantly reduce membrane fouling in ORFBs. These include optimized flow patterns that create turbulence near membrane surfaces, specialized cell architectures that minimize dead zones, and integrated filtration systems that remove potential foulants before they reach the membrane. These engineering approaches maintain consistent electrolyte flow across membrane surfaces, reducing the accumulation of organic deposits.
02 Selective ion-exchange membranes for fouling reduction
Specialized ion-exchange membranes with enhanced selectivity can significantly reduce fouling in aqueous organic redox flow batteries. These membranes are designed to allow specific ion transport while blocking larger organic molecules that cause fouling. By incorporating specific functional groups or adjusting the membrane's pore size distribution, the selective permeability can be optimized to maintain efficient ion transport while minimizing crossover of redox-active organic species that contribute to membrane fouling.Expand Specific Solutions03 In-situ cleaning and regeneration methods
In-situ cleaning and regeneration methods can be implemented to control membrane fouling in organic redox flow batteries without disassembling the system. These methods include periodic electrolyte flushing with cleaning agents, reverse current operation, pH cycling, or pulsed electric field treatments. Such approaches help to dislodge and remove organic foulants from the membrane surface, restoring performance and extending operational lifetime of the battery system.Expand Specific Solutions04 Composite membranes with fouling-resistant layers
Composite membrane structures featuring multiple functional layers can effectively control fouling in aqueous organic redox flow batteries. These membranes typically combine a selective ion-transport layer with one or more protective layers designed to resist organic fouling. The protective layers may incorporate nanoparticles, hydrophilic polymers, or zwitterionic materials that create an unfavorable environment for organic molecule adsorption while maintaining the ion conductivity necessary for battery operation.Expand Specific Solutions05 Electrolyte additives for membrane fouling prevention
Specific additives can be incorporated into the electrolyte solution to prevent membrane fouling in organic redox flow batteries. These additives may include chelating agents, surfactants, or compounds that compete with fouling species for membrane binding sites. By modifying the electrolyte chemistry, these additives help maintain membrane performance by preventing organic molecules from adhering to the membrane surface or by facilitating their removal during normal battery operation.Expand Specific Solutions
Leading Companies and Research Institutions in ORFB Development
The membrane selection and fouling control in aqueous organic redox flow batteries (ORFBs) market is in an early growth phase, with increasing research focus due to its critical role in energy storage applications. The global market is expanding as renewable energy integration drives demand for efficient storage solutions. Technologically, the field is advancing rapidly but still faces challenges in membrane durability and fouling prevention. Leading research institutions like MIT, Rensselaer Polytechnic Institute, and King Abdullah University of Science & Technology are driving academic innovation, while companies such as Asahi Kasei, Xerox Holdings, and Suqian Times Energy Storage Technology are commercializing solutions. The competitive landscape features collaboration between academic institutions and industry players to overcome technical barriers and improve membrane performance for large-scale ORFB deployment.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced ion-selective membranes specifically engineered for aqueous organic redox flow batteries (ORFBs). Their approach focuses on creating nanoporous membranes with precisely controlled pore sizes and surface chemistry to enhance ion selectivity while minimizing crossover of active organic molecules. MIT researchers have pioneered the use of layer-by-layer assembly techniques to create composite membranes with tunable properties. Their membranes incorporate specialized polymer layers that can be adjusted to match the specific redox couples used in the battery system. Additionally, MIT has developed innovative anti-fouling surface treatments that incorporate zwitterionic polymers to create highly hydrophilic surfaces that resist organic molecule adsorption, significantly extending membrane operational lifetime in ORFB applications[1][3]. Their research also includes the development of self-cleaning membrane systems that can periodically reverse ion flow to dislodge accumulated foulants.
Strengths: Superior ion selectivity with minimal crossover, excellent chemical stability in acidic and alkaline environments, and innovative self-cleaning capabilities. Weaknesses: Higher manufacturing complexity and cost compared to commercial membranes, potential challenges in scaling up the layer-by-layer assembly process for industrial production.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei has developed commercial-scale ion exchange membranes specifically optimized for aqueous ORFB applications. Their flagship product line includes modified hydrocarbon and fluorocarbon-based membranes with specialized surface treatments to minimize organic molecule adsorption. The company employs a proprietary manufacturing process that creates asymmetric membrane structures with different pore sizes and surface properties on each side, optimizing the balance between ion conductivity and selectivity. Asahi Kasei's membranes incorporate specialized functional groups that create strong hydration layers, effectively preventing organic molecule adsorption while maintaining high ionic conductivity[4]. Their technology includes integrated anti-fouling additives that are gradually released during operation, providing continuous protection against membrane fouling. Additionally, they've developed specialized membrane conditioning protocols that significantly improve initial performance and reduce break-in time for ORFB systems. Their membranes are manufactured with reinforcement layers that enhance mechanical stability under the pressure differentials commonly experienced in flow battery systems.
Strengths: Mass production capability with consistent quality control, excellent mechanical durability under real-world operating conditions, and optimized balance between performance and cost. Weaknesses: Less customizable than research-grade membranes, potential limitations with certain organic electrolytes, and moderate performance trade-offs to achieve manufacturing scalability.
Key Innovations in Anti-Fouling Membrane Materials
Crossover resistant materials for aqueous organic redox flow batteries
PatentInactiveUS20230275251A1
Innovation
- A redox flow battery design that incorporates a positive electrode electrolyte with a first organic compound and its reduction product, a negative electrode electrolyte with a second redox couple, and an ion exchange membrane that impedes crossover, utilizing membranes with low water content and molecule-membrane combinations where the acidity of the redox molecule is stronger than the membrane's acid groups to restrict crossover.
Materials for use in an aqueous organic redox flow battery
PatentActiveUS10934258B2
Innovation
- The development of aqueous organic redox flow batteries utilizing metallocene- and viologen-based redox active materials, which are highly soluble in aqueous solutions and demonstrate advantageous electrochemical properties, enabling efficient energy storage with sustainable and non-corrosive electrolytes based on earth-abundant elements.
Environmental Impact and Sustainability of ORFB Membrane Materials
The environmental impact of membrane materials in aqueous organic redox flow batteries (ORFBs) represents a critical consideration for the technology's long-term sustainability. Current membrane materials, predominantly perfluorinated sulfonic acid polymers like Nafion, pose significant environmental concerns due to their production processes that involve fluorinated compounds with high global warming potential. The manufacturing of these membranes requires energy-intensive procedures and hazardous chemicals, contributing to considerable carbon footprints and potential environmental contamination.
Life cycle assessments of ORFB membrane materials reveal that their environmental impact extends beyond production to disposal phases. The non-biodegradable nature of fluoropolymer membranes results in persistent environmental presence, with potential for microplastic formation and ecosystem disruption. Additionally, the extraction of raw materials for membrane production often involves mining activities that can lead to habitat destruction and water pollution.
Recent sustainability initiatives have focused on developing alternative membrane materials with reduced environmental footprints. Bio-based polymers derived from renewable resources show promise as environmentally friendly alternatives to traditional fluoropolymer membranes. These materials, including cellulose derivatives and chitosan-based composites, offer biodegradability while maintaining acceptable ion selectivity and conductivity properties essential for ORFB operation.
Water consumption represents another significant environmental consideration in membrane manufacturing. Traditional membrane production processes require substantial water resources for synthesis and purification steps. Innovative water recycling systems and closed-loop manufacturing processes are being implemented to minimize freshwater consumption and reduce wastewater discharge associated with membrane production.
Energy efficiency improvements in membrane manufacturing have demonstrated potential for reducing the overall environmental impact of ORFBs. Advanced production techniques, such as solvent-free processing and low-temperature curing methods, significantly decrease energy requirements while minimizing hazardous waste generation. These approaches align with green chemistry principles and contribute to more sustainable membrane production pathways.
Regulatory frameworks increasingly influence membrane material selection, with restrictions on perfluorinated compounds driving research toward environmentally benign alternatives. The European Union's REACH regulations and similar global initiatives have accelerated the transition away from environmentally persistent materials, encouraging innovation in sustainable membrane development for next-generation ORFBs.
Life cycle assessments of ORFB membrane materials reveal that their environmental impact extends beyond production to disposal phases. The non-biodegradable nature of fluoropolymer membranes results in persistent environmental presence, with potential for microplastic formation and ecosystem disruption. Additionally, the extraction of raw materials for membrane production often involves mining activities that can lead to habitat destruction and water pollution.
Recent sustainability initiatives have focused on developing alternative membrane materials with reduced environmental footprints. Bio-based polymers derived from renewable resources show promise as environmentally friendly alternatives to traditional fluoropolymer membranes. These materials, including cellulose derivatives and chitosan-based composites, offer biodegradability while maintaining acceptable ion selectivity and conductivity properties essential for ORFB operation.
Water consumption represents another significant environmental consideration in membrane manufacturing. Traditional membrane production processes require substantial water resources for synthesis and purification steps. Innovative water recycling systems and closed-loop manufacturing processes are being implemented to minimize freshwater consumption and reduce wastewater discharge associated with membrane production.
Energy efficiency improvements in membrane manufacturing have demonstrated potential for reducing the overall environmental impact of ORFBs. Advanced production techniques, such as solvent-free processing and low-temperature curing methods, significantly decrease energy requirements while minimizing hazardous waste generation. These approaches align with green chemistry principles and contribute to more sustainable membrane production pathways.
Regulatory frameworks increasingly influence membrane material selection, with restrictions on perfluorinated compounds driving research toward environmentally benign alternatives. The European Union's REACH regulations and similar global initiatives have accelerated the transition away from environmentally persistent materials, encouraging innovation in sustainable membrane development for next-generation ORFBs.
Techno-Economic Assessment of Membrane Solutions for ORFBs
The economic viability of organic redox flow batteries (ORFBs) heavily depends on membrane technology, which represents a significant portion of the total system cost. Current membrane solutions for ORFBs face a critical trade-off between performance and cost that directly impacts the commercial feasibility of these energy storage systems. Ion-exchange membranes, particularly perfluorinated membranes like Nafion, offer excellent ionic conductivity and chemical stability but come at prohibitively high costs ranging from $300-500/m². This cost factor alone can contribute up to 40% of the total ORFB stack cost.
Alternative membrane materials such as hydrocarbon-based and composite membranes present more economical options at $50-200/m², but often sacrifice performance metrics including ionic selectivity and long-term durability. The economic assessment must consider not only the upfront material costs but also the lifetime operational expenses related to capacity fade due to crossover and membrane degradation, which can significantly impact the levelized cost of storage (LCOS).
Membrane fouling introduces additional economic considerations, as regular maintenance and cleaning protocols become necessary operational expenses. The cost of downtime during cleaning cycles, replacement of fouled membranes, and chemical cleaning agents must be factored into the total cost of ownership. Studies indicate that effective fouling mitigation strategies can extend membrane lifetime by 30-50%, potentially reducing the annualized membrane replacement costs by 20-30%.
Scale-up considerations reveal that membrane manufacturing processes significantly influence the final cost structure. Roll-to-roll processing of non-fluorinated membranes offers potential cost reductions of up to 60% compared to batch processing methods commonly used for specialized membranes. Additionally, the economy of scale in membrane production could potentially reduce costs by 15-25% when production volumes increase from laboratory to industrial scale.
Sensitivity analysis of ORFB economics demonstrates that membrane selectivity improvements have a greater economic impact than conductivity enhancements. A 10% improvement in selectivity can reduce the LCOS by approximately 7-9%, while a similar improvement in conductivity yields only a 3-5% reduction. This insight should guide research priorities toward selectivity-focused membrane development.
The techno-economic assessment must also consider emerging membrane technologies, such as size-exclusion membranes and functionalized porous separators, which promise cost reductions of 40-60% compared to traditional ion-exchange membranes while maintaining acceptable performance metrics. These alternatives could potentially shift the economic landscape of ORFB technology, bringing the overall system costs closer to the Department of Energy's target of $150/kWh for grid-scale energy storage.
Alternative membrane materials such as hydrocarbon-based and composite membranes present more economical options at $50-200/m², but often sacrifice performance metrics including ionic selectivity and long-term durability. The economic assessment must consider not only the upfront material costs but also the lifetime operational expenses related to capacity fade due to crossover and membrane degradation, which can significantly impact the levelized cost of storage (LCOS).
Membrane fouling introduces additional economic considerations, as regular maintenance and cleaning protocols become necessary operational expenses. The cost of downtime during cleaning cycles, replacement of fouled membranes, and chemical cleaning agents must be factored into the total cost of ownership. Studies indicate that effective fouling mitigation strategies can extend membrane lifetime by 30-50%, potentially reducing the annualized membrane replacement costs by 20-30%.
Scale-up considerations reveal that membrane manufacturing processes significantly influence the final cost structure. Roll-to-roll processing of non-fluorinated membranes offers potential cost reductions of up to 60% compared to batch processing methods commonly used for specialized membranes. Additionally, the economy of scale in membrane production could potentially reduce costs by 15-25% when production volumes increase from laboratory to industrial scale.
Sensitivity analysis of ORFB economics demonstrates that membrane selectivity improvements have a greater economic impact than conductivity enhancements. A 10% improvement in selectivity can reduce the LCOS by approximately 7-9%, while a similar improvement in conductivity yields only a 3-5% reduction. This insight should guide research priorities toward selectivity-focused membrane development.
The techno-economic assessment must also consider emerging membrane technologies, such as size-exclusion membranes and functionalized porous separators, which promise cost reductions of 40-60% compared to traditional ion-exchange membranes while maintaining acceptable performance metrics. These alternatives could potentially shift the economic landscape of ORFB technology, bringing the overall system costs closer to the Department of Energy's target of $150/kWh for grid-scale energy storage.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!