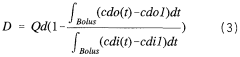How to Develop Cost-Effective Hypertonic Solutions for Broader Use?
Hypertonic Solutions Background and Objectives
Hypertonic solutions have been a cornerstone in medical treatments for decades, primarily used in critical care settings to manage various conditions such as cerebral edema, increased intracranial pressure, and severe hyponatremia. These solutions, characterized by their higher solute concentration compared to bodily fluids, have traditionally been limited in their application due to high production costs and potential side effects.
The evolution of hypertonic solutions can be traced back to the early 20th century when researchers first recognized the potential of using concentrated saline solutions to treat shock and dehydration. Over time, the formulations have been refined, and their applications have expanded, yet the fundamental challenge of cost-effectiveness has persisted, limiting their broader use in healthcare settings.
In recent years, there has been a growing interest in developing more cost-effective hypertonic solutions to expand their utility beyond critical care. This interest is driven by the recognition of their potential benefits in various medical fields, including emergency medicine, sports medicine, and even veterinary care. The primary goal is to create hypertonic solutions that maintain their therapeutic efficacy while significantly reducing production costs, thereby making them more accessible for a wider range of medical applications.
The current technological landscape presents both challenges and opportunities in this pursuit. Advancements in pharmaceutical manufacturing processes, novel delivery systems, and a better understanding of osmotic principles have opened new avenues for innovation. Researchers are exploring alternative solutes, optimizing production methods, and investigating synergistic combinations of ingredients to enhance the cost-effectiveness of hypertonic solutions.
Moreover, the global healthcare landscape is evolving, with an increasing emphasis on outpatient care and home-based treatments. This shift creates a demand for hypertonic solutions that are not only cost-effective but also safe and easy to administer outside of hospital settings. The development of such solutions could revolutionize the management of chronic conditions and improve patient outcomes while reducing healthcare costs.
As we look towards the future, the objectives for developing cost-effective hypertonic solutions are multifaceted. They include reducing production costs without compromising quality, expanding the range of medical conditions that can be effectively treated with these solutions, and improving their safety profile to enable broader use. Additionally, there is a focus on developing formulations that are stable at room temperature and have longer shelf lives, further enhancing their practicality and cost-effectiveness.
Market Analysis for Hypertonic Solutions
The market for hypertonic solutions is experiencing significant growth, driven by increasing applications in healthcare, agriculture, and industrial processes. In the healthcare sector, hypertonic solutions are widely used for treating various medical conditions, including edema, intracranial pressure, and dehydration. The global market for medical-grade hypertonic solutions is projected to expand steadily, with a particular focus on developing countries where access to advanced healthcare treatments is improving.
In agriculture, hypertonic solutions play a crucial role in seed priming and soil treatment, enhancing crop yields and stress resistance. The agricultural sector's demand for cost-effective hypertonic solutions is rising, especially in regions facing water scarcity and soil salinity issues. This trend is expected to continue as farmers seek innovative methods to improve crop productivity and sustainability.
Industrial applications of hypertonic solutions, such as in food processing, water treatment, and chemical manufacturing, are also contributing to market growth. The increasing emphasis on water conservation and efficient resource utilization in industrial processes is driving the adoption of hypertonic solution technologies.
The market landscape is characterized by a mix of established players and emerging companies focusing on developing innovative, cost-effective formulations. Key market segments include sodium chloride-based solutions, glucose-based solutions, and multi-electrolyte formulations. While sodium chloride solutions dominate the market due to their widespread use and low cost, there is growing interest in more complex formulations that offer additional benefits.
Geographically, North America and Europe currently lead the market, owing to advanced healthcare infrastructure and high R&D investments. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing healthcare expenditure, agricultural modernization, and industrial growth in countries like China and India.
A significant market trend is the shift towards more sustainable and environmentally friendly hypertonic solutions. This includes the development of bio-based formulations and recyclable packaging solutions. Additionally, there is a growing demand for personalized hypertonic solutions in healthcare, tailored to individual patient needs.
The market faces challenges such as stringent regulatory requirements, especially for medical-grade solutions, and the need for continuous innovation to improve efficacy and reduce costs. However, these challenges also present opportunities for companies to differentiate themselves through advanced research and development efforts.
In conclusion, the market for hypertonic solutions shows promising growth potential across various sectors. The key to success in this market lies in developing cost-effective, versatile formulations that can address a wide range of applications while meeting regulatory standards and sustainability goals.
Technical Challenges in Hypertonic Solution Development
The development of cost-effective hypertonic solutions faces several significant technical challenges. One of the primary obstacles is achieving the desired osmolality while maintaining solution stability. Hypertonic solutions require a high concentration of solutes, which can lead to issues with solubility and precipitation. Ensuring that all components remain in solution over extended periods and under various storage conditions is crucial for product efficacy and safety.
Another challenge lies in the selection of appropriate solutes. While sodium chloride is commonly used, it may not be suitable for all applications. Identifying alternative solutes that can provide the necessary osmotic effect without adverse reactions or interactions with other components is an ongoing area of research. This is particularly important when developing hypertonic solutions for specific medical treatments or industrial processes.
The manufacturing process itself presents technical hurdles. Achieving precise and consistent osmolality across batches requires sophisticated equipment and rigorous quality control measures. The production environment must be carefully controlled to prevent contamination and ensure sterility, especially for medical-grade solutions. Scaling up production while maintaining quality and cost-effectiveness is a significant challenge that requires innovative approaches to process engineering.
Packaging and storage of hypertonic solutions also pose technical difficulties. The high solute concentration can potentially interact with packaging materials, leading to leaching or degradation. Developing packaging that is both cost-effective and capable of maintaining the integrity of the solution over its shelf life is a critical area of focus. Additionally, some hypertonic solutions may require special storage conditions to prevent crystallization or bacterial growth, adding complexity to the supply chain.
Formulation stability is another key challenge. Hypertonic solutions must maintain their osmolality and chemical composition throughout their shelf life. This requires careful selection of preservatives and stabilizers that are compatible with the high solute concentration and do not interfere with the solution's intended use. Balancing stability with cost-effectiveness often involves trade-offs that must be carefully evaluated.
Lastly, the development of analytical methods for quality control and characterization of hypertonic solutions presents its own set of challenges. Standard techniques may not be suitable for high-concentration solutions, necessitating the development of specialized testing protocols. Ensuring accurate and reproducible measurements of osmolality, pH, and chemical composition is essential for regulatory compliance and product consistency.
Current Cost-Effective Hypertonic Solution Approaches
01 Cost-effective formulation of hypertonic solutions
Developing cost-effective formulations for hypertonic solutions involves optimizing the composition and manufacturing process. This includes selecting affordable ingredients, streamlining production methods, and improving stability to extend shelf life. Such approaches can significantly reduce production costs while maintaining therapeutic efficacy.- Cost-effective formulation of hypertonic solutions: Developing cost-effective formulations for hypertonic solutions involves optimizing the composition and manufacturing process. This includes selecting affordable ingredients, streamlining production methods, and improving stability to extend shelf life. Such approaches can reduce overall costs while maintaining therapeutic efficacy.
- Economic analysis of hypertonic solution usage: Conducting economic analyses to evaluate the cost-effectiveness of hypertonic solutions in various medical applications. This involves comparing treatment outcomes, resource utilization, and overall healthcare costs associated with hypertonic solutions versus alternative therapies.
- Efficient delivery systems for hypertonic solutions: Developing innovative delivery systems for hypertonic solutions to improve administration efficiency and reduce waste. This may include advanced infusion devices, precise dosing mechanisms, or novel packaging solutions that minimize product loss and optimize usage.
- Optimization of hypertonic solution production processes: Implementing advanced manufacturing techniques and process optimizations to enhance the production efficiency of hypertonic solutions. This may involve automation, continuous flow processes, or other innovative approaches to reduce production costs and improve scalability.
- Quality control and cost reduction in hypertonic solutions: Developing and implementing robust quality control measures for hypertonic solutions while simultaneously reducing costs. This includes utilizing advanced analytical techniques, implementing risk-based approaches, and optimizing testing protocols to ensure product quality and safety without unnecessary expenses.
02 Economic analysis of hypertonic solution treatments
Conducting economic analyses to evaluate the cost-effectiveness of hypertonic solution treatments in various medical applications. This involves comparing the costs and outcomes of hypertonic solutions with alternative treatments, considering factors such as efficacy, duration of treatment, and potential complications.Expand Specific Solutions03 Innovative delivery systems for hypertonic solutions
Developing innovative delivery systems for hypertonic solutions to improve efficiency and reduce waste. This may include novel packaging designs, precise dosing mechanisms, or controlled-release formulations that optimize the use of the solution and minimize the amount required for effective treatment.Expand Specific Solutions04 Automation and process optimization in production
Implementing automation and process optimization techniques in the production of hypertonic solutions to increase efficiency and reduce costs. This may involve using advanced manufacturing technologies, real-time monitoring systems, and data analytics to streamline production processes and minimize resource consumption.Expand Specific Solutions05 Sustainable and eco-friendly production methods
Developing sustainable and eco-friendly production methods for hypertonic solutions to reduce environmental impact and associated costs. This includes using renewable resources, implementing energy-efficient processes, and minimizing waste generation throughout the production and packaging stages.Expand Specific Solutions
Key Players in Hypertonic Solution Industry
The development of cost-effective hypertonic solutions for broader use is in a growth phase, with increasing market demand and technological advancements. The global market for hypertonic solutions is expanding, driven by rising applications in healthcare and industrial sectors. Technological maturity varies among key players, with established pharmaceutical companies like Janssen Pharmaceutica NV, B. Braun Melsungen AG, and Fresenius Medical Care Deutschland GmbH leading in innovation. Academic institutions such as the University of South Carolina and Zhejiang University contribute to research advancements. Emerging players like Hanmi Pharmaceutical Co., Ltd. and Xeris Pharmaceuticals, Inc. are introducing novel approaches, while companies like Cellphire, Inc. focus on specialized applications. The competitive landscape is characterized by a mix of established firms and innovative startups, driving progress in formulation techniques and cost-reduction strategies.
Janssen Pharmaceutica NV
B. Braun Melsungen AG
Innovative Formulations for Hypertonic Solutions
- A resuscitation fluid comprising a hypertonic ionic salt, a soluble protein like albumin, and an intermediate energy substrate like pyruvate, optionally with an agent to mitigate intracellular acidosis, is administered at a lower volume to effectively transfer interstitial fluid into the vascular space, maintaining osmotic pressure and aerobic metabolism while minimizing inflammatory responses.
- A method involving a bolus of hypertonic NaCl solution is introduced to rapidly change the dialysis fluid inlet concentration, allowing the calculation of dialysance through integration of concentration differences and fluid flow rates, eliminating the need for constant inlet concentration settings and reducing measurement time.
Regulatory Considerations for Hypertonic Solutions
The regulatory landscape for hypertonic solutions is complex and multifaceted, requiring careful navigation to ensure compliance and market access. In the United States, the Food and Drug Administration (FDA) oversees the regulation of hypertonic solutions, classifying them based on their intended use and composition. For medical applications, hypertonic solutions are typically regulated as drugs or medical devices, depending on their specific formulation and purpose.
Key considerations in the regulatory process include safety, efficacy, and quality control. Manufacturers must demonstrate through rigorous clinical trials that their hypertonic solutions are safe for use and effective in achieving their intended purpose. This often involves extensive documentation, including detailed information on the manufacturing process, quality control measures, and stability testing.
The FDA's approval process for hypertonic solutions can be lengthy and costly, particularly for novel formulations or applications. However, there are pathways for expedited review in cases where the solution addresses an unmet medical need or offers significant advantages over existing treatments. Companies developing cost-effective hypertonic solutions should consider these pathways as potential strategies for faster market entry.
Internationally, regulatory requirements for hypertonic solutions can vary significantly. The European Medicines Agency (EMA) in the European Union, for instance, has its own set of guidelines and approval processes. Companies aiming for global distribution must navigate these diverse regulatory environments, often necessitating separate approval processes for different markets.
Labeling and packaging requirements are another critical aspect of regulatory compliance for hypertonic solutions. Clear, accurate labeling that includes indications, contraindications, and proper usage instructions is essential. For solutions intended for broader use, particularly in non-medical settings, labeling must be easily understandable by the general public.
Post-market surveillance is an ongoing regulatory requirement for hypertonic solutions. Manufacturers must monitor their products for adverse events and report any safety concerns to regulatory authorities. This continuous monitoring helps ensure the long-term safety and efficacy of the solutions in real-world use.
As the development of cost-effective hypertonic solutions progresses, regulatory strategies should be integrated early in the product development process. This proactive approach can help identify potential regulatory hurdles and streamline the path to market approval. Collaboration with regulatory experts and early engagement with regulatory bodies can provide valuable insights and guidance, potentially reducing development costs and timelines.
Environmental Impact of Hypertonic Solution Production
The production of hypertonic solutions, while essential for various applications, carries significant environmental implications that warrant careful consideration. The manufacturing process often involves energy-intensive operations, contributing to greenhouse gas emissions and climate change. Water consumption is another critical factor, as large volumes are required for solution preparation and equipment cleaning, potentially straining local water resources in areas of production.
Chemical inputs used in hypertonic solution production can lead to environmental contamination if not properly managed. Salts, sugars, and other solutes may enter waterways through improper disposal or accidental spills, affecting aquatic ecosystems and potentially harming wildlife. Additionally, the packaging materials used for hypertonic solutions, often plastic containers or bags, contribute to the global plastic waste problem if not recycled or disposed of responsibly.
Transportation of raw materials and finished products also adds to the environmental footprint through fuel consumption and emissions. This impact is particularly pronounced when ingredients or solutions are shipped over long distances. Furthermore, the energy required for storage, especially for temperature-sensitive solutions, can be substantial, contributing to overall energy consumption and associated environmental costs.
Efforts to mitigate these environmental impacts are crucial for developing more sustainable hypertonic solution production methods. Implementing closed-loop water systems can significantly reduce water consumption and minimize wastewater discharge. Adopting renewable energy sources for manufacturing processes and transportation can help decrease carbon emissions. Innovations in packaging, such as using biodegradable or recyclable materials, can address plastic waste concerns.
Research into more environmentally friendly solutes and production methods is ongoing. This includes exploring natural, biodegradable alternatives to traditional chemical ingredients and developing more efficient production techniques that reduce energy and resource consumption. Additionally, optimizing supply chains to minimize transportation distances and implementing just-in-time production strategies can further reduce the environmental impact of hypertonic solution manufacturing and distribution.
As the demand for hypertonic solutions grows across various sectors, including healthcare, agriculture, and food preservation, addressing these environmental challenges becomes increasingly important. Balancing cost-effectiveness with environmental responsibility will be key to developing sustainable production practices that meet market needs while minimizing ecological harm.