How to Ensure Precision in Hypertonic Solution Manufacturing?
Hypertonic Solution Precision Manufacturing Background
Hypertonic solutions have been a critical component in various medical and industrial applications for decades. These solutions, characterized by their higher solute concentration compared to the surrounding environment, play vital roles in diverse fields such as pharmaceuticals, biotechnology, and food processing. The precision in manufacturing hypertonic solutions has become increasingly important due to their widespread use and the potential consequences of inaccuracies.
The evolution of hypertonic solution manufacturing has been closely tied to advancements in chemical engineering, analytical techniques, and quality control processes. Initially, the production of these solutions relied heavily on manual methods, which were prone to human error and inconsistencies. As technology progressed, automated systems and more sophisticated measurement tools were introduced, significantly improving the accuracy and reproducibility of hypertonic solution preparation.
The primary goal in hypertonic solution manufacturing is to achieve and maintain precise solute concentrations. This precision is crucial for several reasons. In medical applications, such as intravenous therapies, even slight deviations in concentration can have serious implications for patient safety and treatment efficacy. In industrial settings, such as food preservation or biotechnology processes, precise hypertonic solutions are essential for maintaining product quality and consistency.
The challenges in ensuring precision have evolved alongside technological advancements. Early issues centered around basic measurement accuracy and mixing techniques. As manufacturing processes became more refined, the focus shifted to addressing more subtle factors affecting precision, such as environmental conditions, raw material variability, and equipment calibration.
Recent technological trends in hypertonic solution manufacturing include the integration of real-time monitoring systems, the use of advanced analytical techniques for quality control, and the implementation of automated feedback loops to maintain precise concentrations throughout the production process. These advancements aim to minimize human intervention and reduce the potential for errors.
The industry has also seen a growing emphasis on standardization and regulatory compliance. Governing bodies in various sectors have established stringent guidelines for the manufacture of hypertonic solutions, particularly those used in medical applications. These regulations have further driven the need for precision and consistency in manufacturing processes.
As we look towards the future, the field of hypertonic solution manufacturing is poised for further innovation. Emerging technologies such as artificial intelligence and machine learning are expected to play significant roles in optimizing production processes and predicting potential issues before they occur. Additionally, there is a growing interest in developing more environmentally sustainable manufacturing methods without compromising precision.
Market Analysis for Hypertonic Solutions
The global market for hypertonic solutions has been experiencing steady growth, driven by increasing prevalence of dehydration-related conditions, rising demand for sports and fitness beverages, and expanding applications in medical treatments. The market size for hypertonic solutions is projected to reach significant value in the coming years, with a compound annual growth rate (CAGR) outpacing many other pharmaceutical segments.
Key factors contributing to market growth include the rising incidence of gastrointestinal disorders, growing awareness about the importance of proper hydration, and the expanding geriatric population. Additionally, the increasing adoption of hypertonic solutions in critical care settings and emergency departments for treating various medical conditions has further bolstered market demand.
The sports and fitness industry represents a substantial portion of the hypertonic solution market, with athletes and fitness enthusiasts increasingly recognizing the benefits of these products for rapid rehydration and electrolyte replenishment. This segment is expected to continue its upward trajectory as health and wellness trends gain momentum globally.
In the medical field, hypertonic solutions are widely used in the treatment of edema, increased intracranial pressure, and as a component in various intravenous therapies. The growing prevalence of chronic diseases and the rise in surgical procedures worldwide have contributed to the increased demand for hypertonic solutions in healthcare settings.
Geographically, North America and Europe currently dominate the hypertonic solution market, owing to advanced healthcare infrastructure, high healthcare expenditure, and greater awareness among consumers. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by improving healthcare facilities, rising disposable incomes, and increasing health consciousness among the population.
The market is characterized by the presence of several key players, including pharmaceutical giants and specialized manufacturers. These companies are focusing on product innovation, expanding their product portfolios, and strategic collaborations to gain a competitive edge. The increasing emphasis on precision manufacturing and quality control in the production of hypertonic solutions is expected to drive further advancements in the industry.
Challenges facing the market include stringent regulatory requirements, particularly for medical-grade hypertonic solutions, and the need for maintaining precise osmolality levels during manufacturing. However, these challenges also present opportunities for companies that can develop innovative manufacturing processes and quality control measures to ensure consistent product precision.
Current Challenges in Hypertonic Solution Production
The production of hypertonic solutions presents several significant challenges that manufacturers must address to ensure precision and quality. One of the primary difficulties lies in maintaining consistent osmolality across batches. Osmolality, a critical parameter in hypertonic solutions, can be affected by various factors during the manufacturing process, including temperature fluctuations, ingredient purity, and mixing techniques. Even minor deviations can lead to solutions that are either ineffective or potentially harmful to patients.
Another challenge is the precise measurement and incorporation of solutes. Hypertonic solutions often require exact quantities of multiple ingredients, and any errors in measurement or addition can significantly impact the final product's composition. This is particularly crucial when dealing with highly concentrated solutions, where small discrepancies can have magnified effects on the overall tonicity.
The stability of hypertonic solutions during storage and transportation also poses a considerable challenge. These solutions can be sensitive to environmental factors such as light, temperature, and humidity. Manufacturers must develop robust packaging and storage protocols to maintain the integrity of the solution throughout its shelf life. This often involves extensive stability testing and the implementation of stringent quality control measures.
Sterility is another critical concern in hypertonic solution production. As these solutions are often used in medical settings, ensuring absolute sterility is paramount. The manufacturing process must incorporate aseptic techniques and validated sterilization methods to eliminate any risk of microbial contamination. This requirement adds complexity to the production process and necessitates specialized equipment and facilities.
Furthermore, the scaling of production from laboratory to industrial levels presents its own set of challenges. Processes that work well at small scales may not translate directly to large-scale manufacturing. Issues such as heat transfer, mixing efficiency, and filtration can become more pronounced as batch sizes increase. Manufacturers must carefully optimize their processes to maintain consistency and quality across different production scales.
Regulatory compliance adds another layer of complexity to hypertonic solution manufacturing. Stringent guidelines set by regulatory bodies such as the FDA and EMA require extensive documentation, validation of processes, and regular audits. Meeting these requirements while maintaining efficiency and cost-effectiveness is a constant challenge for manufacturers.
Lastly, the variability in raw materials can significantly impact the precision of hypertonic solution production. Ingredients sourced from different suppliers or even different batches from the same supplier may have slight variations in purity or composition. This variability necessitates robust quality control processes and may require adjustments to formulations to ensure consistent end-product quality.
Existing Precision Control Methods
01 Hypertonic solution formulation
Hypertonic solutions are formulated with precise concentrations of solutes to create an osmotic gradient. These solutions are used in various medical and biological applications, including cell preservation, wound treatment, and diagnostic procedures. The precision in formulation is crucial to ensure the desired osmotic effect and maintain the integrity of cells or tissues.- Hypertonic solution formulation for medical applications: Hypertonic solutions are developed for various medical applications, including wound healing, osmotic therapy, and treatment of edema. These solutions have a higher solute concentration than body fluids, creating an osmotic gradient that can draw fluid out of tissues or cells. Precise formulation of these solutions is crucial for their effectiveness and safety in medical use.
- Precision control in hypertonic solution preparation: Achieving precision in hypertonic solution preparation involves careful control of solute concentration, pH, and osmolality. Advanced techniques and equipment are employed to ensure accurate measurements and consistent quality. This precision is essential for maintaining the desired therapeutic effects and minimizing potential side effects in clinical applications.
- Hypertonic solutions for specific medical conditions: Tailored hypertonic solutions are developed for specific medical conditions such as cerebral edema, shock, and electrolyte imbalances. These solutions are precisely formulated to address the unique requirements of each condition, optimizing their therapeutic efficacy while minimizing potential adverse effects.
- Novel delivery systems for hypertonic solutions: Innovative delivery systems are designed to enhance the precision and effectiveness of hypertonic solution administration. These may include controlled-release mechanisms, targeted delivery methods, or specialized devices that ensure accurate dosing and optimal distribution of the solution within the body.
- Quality control and stability of hypertonic solutions: Ensuring the long-term stability and maintaining the precise composition of hypertonic solutions is crucial for their efficacy and safety. Advanced quality control measures, storage techniques, and packaging innovations are developed to preserve the integrity of these solutions throughout their shelf life and during administration.
02 Osmolarity measurement techniques
Accurate measurement of osmolarity is essential for ensuring the precision of hypertonic solutions. Various techniques and devices are employed to measure osmolarity, including freezing point depression, vapor pressure osmometry, and membrane osmometry. These methods allow for precise determination of solute concentration and osmotic pressure in hypertonic solutions.Expand Specific Solutions03 Applications in cell culture and preservation
Hypertonic solutions play a crucial role in cell culture and preservation techniques. Precise formulations are used to control cell volume, maintain cellular integrity, and optimize growth conditions. These solutions are also employed in cryopreservation protocols to protect cells from damage during freezing and thawing processes.Expand Specific Solutions04 Medical applications of hypertonic solutions
Hypertonic solutions have various medical applications, including treatment of edema, intracranial pressure management, and wound healing. The precision of these solutions is critical for their effectiveness and safety in clinical settings. Specialized formulations are developed for specific medical conditions and routes of administration.Expand Specific Solutions05 Quality control and stability testing
Ensuring the precision and stability of hypertonic solutions requires rigorous quality control measures. Analytical techniques are employed to verify the concentration of solutes, pH, and osmolarity. Stability testing protocols are developed to assess the long-term integrity of hypertonic solutions under various storage conditions.Expand Specific Solutions
Key Players in Pharmaceutical Manufacturing
The hypertonic solution manufacturing industry is in a mature stage, with a growing market size driven by increasing demand in healthcare and biotechnology sectors. The technology for ensuring precision in manufacturing has reached a high level of maturity, with several key players contributing to advancements. Companies like Merck Patent GmbH, Fresenius Medical Care Deutschland GmbH, and Norton Healthcare, Inc. are at the forefront, leveraging their expertise in pharmaceutical and medical technologies. Academic institutions such as Tongji University, Fudan University, and Shandong University are also contributing to research and innovation in this field. The competitive landscape is characterized by a mix of established pharmaceutical companies and specialized solution manufacturers, with ongoing efforts to improve precision, efficiency, and quality control in hypertonic solution production.
Merck Patent GmbH
Air Liquide Electronics Systems SA
Innovative Technologies for Osmolality Control
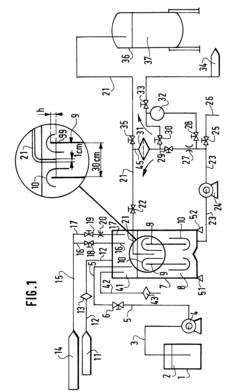
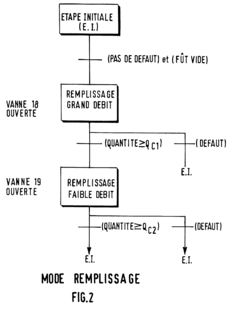
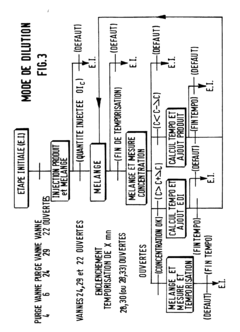
- A method using a piston pump with an adjustable piston and temperature monitoring to calculate the required delivery volume based on fluid density, accounting for gas content, and implementing a dosing system with separate lines and valves to prevent mixing errors, ensuring precise dosing by filling the working space with excess volume and draining excess fluid.
- A system that uses a first container for concentrated chemicals and a source of ultra-pure water to mix and adjust the concentration, ensuring homogenization through recirculation and precise measurement of the diluted solution's titer, allowing for the addition of more water or chemicals to achieve the desired concentration, with a closed tank and non-contact density measurement for accuracy.
Quality Control and Regulatory Compliance
Quality control and regulatory compliance are paramount in ensuring precision in hypertonic solution manufacturing. Strict adherence to Good Manufacturing Practices (GMP) is essential to maintain consistent product quality and meet regulatory requirements. Manufacturers must implement robust quality management systems that encompass all aspects of production, from raw material sourcing to final product release.
A critical component of quality control is the establishment of Standard Operating Procedures (SOPs) for each step of the manufacturing process. These SOPs should detail precise protocols for solution preparation, including weighing, mixing, and sterilization procedures. Regular calibration and maintenance of equipment, particularly precision instruments like balances and osmometers, are crucial for accurate measurements and consistent product quality.
In-process controls play a vital role in maintaining precision throughout the manufacturing process. This includes regular sampling and testing of the solution at various stages to ensure that parameters such as osmolality, pH, and concentration remain within specified limits. Implementation of real-time monitoring systems can provide continuous data on critical process parameters, allowing for immediate corrective actions if deviations occur.
Regulatory compliance in hypertonic solution manufacturing involves adherence to guidelines set by authorities such as the FDA, EMA, and WHO. These regulations often require validation of manufacturing processes, equipment qualification, and thorough documentation of all production activities. Manufacturers must conduct regular internal audits and be prepared for inspections by regulatory bodies to ensure ongoing compliance.
Quality control laboratories play a crucial role in ensuring precision. They must be equipped with state-of-the-art analytical instruments and staffed by qualified personnel. Rigorous testing of raw materials, in-process samples, and finished products is essential to verify that all specifications are met consistently. The use of validated analytical methods and participation in proficiency testing programs can further enhance the reliability of test results.
Traceability is another key aspect of quality control and regulatory compliance. Implementing a comprehensive batch record system allows for the tracking of all materials, processes, and personnel involved in each production run. This not only aids in quality assurance but also facilitates efficient recall procedures if necessary.
Employee training and competency assessment are integral to maintaining precision in manufacturing. Regular training programs should be conducted to ensure that all staff members are up-to-date with current GMP requirements, SOPs, and the latest technological advancements in hypertonic solution production. Periodic evaluation of employee performance helps identify areas for improvement and ensures consistent adherence to quality standards.
Environmental Impact of Manufacturing Processes
The manufacturing of hypertonic solutions, while crucial for various medical and industrial applications, can have significant environmental impacts that need to be carefully considered and mitigated. The production process often involves the use of chemicals, energy-intensive operations, and the generation of waste products, all of which can contribute to environmental degradation if not properly managed.
One of the primary environmental concerns in hypertonic solution manufacturing is water consumption and wastewater generation. The production process typically requires large volumes of purified water, which can strain local water resources, especially in water-scarce regions. Additionally, the wastewater produced during manufacturing may contain high concentrations of salts, chemicals, and other contaminants that can harm aquatic ecosystems if not adequately treated before discharge.
Energy consumption is another significant environmental factor in the production of hypertonic solutions. The processes involved, such as heating, cooling, and sterilization, often require substantial amounts of energy, contributing to greenhouse gas emissions and climate change. Implementing energy-efficient technologies and renewable energy sources can help reduce the carbon footprint of manufacturing facilities.
Chemical usage and disposal present further environmental challenges. Many hypertonic solutions require the use of various chemicals, some of which may be hazardous or toxic. Proper handling, storage, and disposal of these substances are essential to prevent soil and groundwater contamination. Additionally, the production of raw materials used in hypertonic solutions may have upstream environmental impacts, including resource extraction and transportation emissions.
Air emissions from manufacturing processes can also contribute to local air quality issues. Volatile organic compounds (VOCs) and particulate matter may be released during production, potentially affecting both human health and the environment. Implementing effective air filtration systems and adopting low-emission technologies can help mitigate these impacts.
Packaging and transportation of hypertonic solutions add to the overall environmental footprint of the product. Single-use plastic containers and packaging materials contribute to plastic waste, while transportation emissions increase the product's carbon footprint. Exploring sustainable packaging alternatives and optimizing distribution networks can help reduce these impacts.
To address these environmental concerns, manufacturers of hypertonic solutions should adopt sustainable practices throughout the production process. This may include implementing closed-loop water systems, investing in energy-efficient equipment, using green chemistry principles, and developing comprehensive waste management strategies. Additionally, conducting regular environmental impact assessments and life cycle analyses can help identify areas for improvement and guide sustainability efforts in the manufacturing process.