How to Formulate Hypertonic Solutions for Cell Dehydration?
Hypertonic Solution Background and Objectives
Hypertonic solutions have been a cornerstone in cell biology and medical applications for decades. These solutions, characterized by their higher solute concentration compared to the intracellular environment, play a crucial role in various biological processes and therapeutic interventions. The primary mechanism of action for hypertonic solutions is osmosis, where water moves from an area of lower solute concentration to an area of higher solute concentration across a semipermeable membrane.
The development of hypertonic solutions for cell dehydration has its roots in early osmotic studies dating back to the 19th century. However, it wasn't until the mid-20th century that researchers began to fully appreciate the potential applications of controlled cell dehydration in fields such as cryopreservation, drug delivery, and tissue engineering. The ability to manipulate cellular water content has proven invaluable in preserving biological samples, enhancing drug efficacy, and modulating cell behavior in various experimental and clinical settings.
As our understanding of cell physiology and membrane dynamics has advanced, so too has the sophistication of hypertonic solution formulations. Modern approaches incorporate a diverse array of solutes, including sugars, salts, and synthetic polymers, each selected for specific physicochemical properties and biological effects. The goal in formulating these solutions extends beyond mere dehydration; researchers now aim to optimize cellular responses, minimize toxicity, and enhance overall efficacy in targeted applications.
The objectives of current research in hypertonic solution formulation are multifaceted. Primarily, there is a drive to develop more precise and controllable methods of cell dehydration, allowing for finer manipulation of cellular water content and solute concentrations. This precision is critical in applications such as cryopreservation, where optimal dehydration can significantly improve cell viability upon thawing. Additionally, researchers are exploring novel solutes and combination strategies to mitigate the potential negative effects of hyperosmotic stress on cells, such as oxidative damage and apoptosis.
Another key objective is the tailoring of hypertonic solutions for specific cell types and tissues. Different cells exhibit varying osmotic tolerances and membrane permeabilities, necessitating customized formulations for optimal results. This personalized approach is particularly relevant in fields like regenerative medicine and organ preservation, where maintaining the integrity and function of complex tissues is paramount.
Furthermore, there is growing interest in developing "smart" hypertonic solutions that can respond dynamically to environmental cues or cellular feedback. These advanced formulations could potentially offer more nuanced control over cell dehydration processes, adapting to the changing needs of cells during experimental procedures or therapeutic interventions. Such innovations promise to expand the applications of hypertonic solutions and enhance their efficacy across a broad spectrum of biological and medical fields.
Market Analysis for Cell Dehydration Applications
The market for cell dehydration applications is experiencing significant growth, driven by advancements in biotechnology, pharmaceutical research, and medical diagnostics. Hypertonic solutions play a crucial role in various cell-based processes, including cryopreservation, osmotic stress studies, and cell volume regulation experiments. The global market for cell culture media, which includes hypertonic solutions, is projected to reach substantial value in the coming years.
Pharmaceutical and biotechnology companies represent the largest segment of end-users for cell dehydration applications. These industries utilize hypertonic solutions in drug discovery processes, toxicity testing, and the development of cell-based therapies. The increasing focus on personalized medicine and regenerative therapies is expected to further boost the demand for advanced cell manipulation techniques, including controlled dehydration.
Academic and research institutions form another significant market segment, as they conduct fundamental research on cell biology, osmotic regulation, and stress responses. The growing interest in understanding cellular mechanisms and developing novel therapeutic approaches drives the demand for high-quality hypertonic solutions in this sector.
The clinical diagnostics market also contributes to the demand for cell dehydration applications. Hypertonic solutions are used in various diagnostic procedures, such as blood cell analysis and the preparation of clinical samples. As healthcare systems worldwide emphasize early disease detection and personalized treatment strategies, the market for advanced diagnostic tools incorporating cell dehydration techniques is expected to expand.
Geographically, North America and Europe currently dominate the market for cell dehydration applications, owing to their well-established research infrastructure and high investment in life sciences. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing research activities, expanding biotechnology sectors, and rising healthcare expenditure in countries like China and India.
Key market trends include the development of chemically defined and animal component-free hypertonic solutions to meet the stringent quality requirements of biopharmaceutical production. There is also a growing demand for ready-to-use, pre-formulated solutions that offer consistency and convenience in research and clinical settings.
The competitive landscape of the cell dehydration market is characterized by the presence of both large multinational corporations and specialized biotechnology companies. Major players are focusing on product innovation, strategic collaborations, and expanding their product portfolios to maintain their market positions and address evolving customer needs.
Current Challenges in Hypertonic Solution Formulation
The formulation of hypertonic solutions for cell dehydration faces several significant challenges in the current scientific and industrial landscape. One of the primary obstacles is achieving precise osmolarity control. Hypertonic solutions require a delicate balance of solute concentration to create the desired osmotic gradient without causing excessive cellular stress or damage. Maintaining this balance consistently across batches and during storage remains a persistent challenge.
Another critical issue is the selection of appropriate solutes. While traditional options like sucrose and sodium chloride are widely used, they may not be suitable for all cell types or applications. Researchers are continually seeking alternative solutes that can provide effective dehydration while minimizing cellular toxicity and preserving cell viability. This search is complicated by the diverse physiological requirements of different cell types and the need for compatibility with downstream processes.
The stability of hypertonic solutions over time poses another significant challenge. Many formulations are prone to degradation, precipitation, or microbial contamination during storage. This instability can lead to inconsistent performance and reduced shelf life, which is particularly problematic for commercial applications and long-term research projects. Developing stable formulations that maintain their hypertonic properties and effectiveness over extended periods is an ongoing area of research and development.
Scalability and cost-effectiveness present additional hurdles in hypertonic solution formulation. While small-scale laboratory preparations may be straightforward, scaling up production for industrial or clinical applications often introduces new complexities. Ensuring homogeneity, sterility, and consistency in large-volume productions can be technically challenging and economically demanding. Balancing the need for high-quality, effective solutions with cost considerations is crucial for widespread adoption and commercial viability.
Furthermore, the biocompatibility of hypertonic solutions remains a significant concern, especially for biomedical applications. Formulations must not only effectively dehydrate cells but also be non-toxic, non-immunogenic, and compatible with biological systems. This requirement is particularly critical in areas such as cryopreservation, where cells must survive both the dehydration process and subsequent rehydration. Developing solutions that meet these stringent biocompatibility criteria while maintaining optimal dehydration efficiency is an ongoing challenge in the field.
Lastly, regulatory compliance adds another layer of complexity to hypertonic solution formulation. Depending on the intended application, particularly in medical or food-related fields, formulations may need to meet strict regulatory standards. Navigating these regulatory requirements while maintaining the solution's effectiveness and economic viability can be a significant challenge for researchers and manufacturers alike.
Existing Hypertonic Solution Formulations
01 Hypertonic solutions for cell dehydration
Hypertonic solutions are used to induce cell dehydration by creating an osmotic gradient across the cell membrane. This process causes water to move out of the cell, leading to shrinkage and dehydration. These solutions are utilized in various biological and medical applications, including cryopreservation and osmotic stress studies.- Hypertonic solutions for cell dehydration: Hypertonic solutions are used to induce cell dehydration by creating an osmotic gradient across the cell membrane. This process causes water to move out of the cell, leading to shrinkage and dehydration. These solutions are utilized in various biological and medical applications, including cryopreservation and osmotic stress studies.
- Cell preservation techniques using hypertonic solutions: Hypertonic solutions play a crucial role in cell preservation techniques, particularly in cryopreservation. By dehydrating cells prior to freezing, these solutions help prevent intracellular ice formation, which can damage cellular structures. This method is widely used in the preservation of various cell types and tissues for research and medical purposes.
- Osmotic stress and cellular response studies: Hypertonic solutions are employed in research to study cellular responses to osmotic stress. By exposing cells to these solutions, researchers can investigate mechanisms of osmoadaptation, stress response pathways, and cellular volume regulation. This research contributes to understanding cellular physiology and potential therapeutic interventions for osmotic imbalances.
- Medical applications of hypertonic solutions: Hypertonic solutions have various medical applications, including treatment of edema, intracranial pressure reduction, and as components in certain medical devices. These solutions can help draw excess fluid from tissues or create specific osmotic environments for therapeutic purposes. Their use extends to areas such as wound care and management of certain medical conditions.
- Development of specialized hypertonic formulations: Research focuses on developing specialized hypertonic formulations for specific applications. These may include optimized solutions for particular cell types, tissues, or medical procedures. Efforts are made to enhance the effectiveness of these solutions while minimizing potential adverse effects on cells or tissues. This involves studying various solutes, concentrations, and additives to achieve desired osmotic effects.
02 Cell preservation techniques using hypertonic solutions
Hypertonic solutions play a crucial role in cell preservation techniques, particularly in cryopreservation. By dehydrating cells prior to freezing, these solutions help prevent intracellular ice formation, which can damage cellular structures. This method is widely used in the preservation of various cell types and tissues for research and medical purposes.Expand Specific Solutions03 Osmotic stress and cellular response studies
Hypertonic solutions are employed in research to study cellular responses to osmotic stress. By exposing cells to these solutions, researchers can investigate mechanisms of osmoadaptation, stress response pathways, and cellular volume regulation. This research contributes to understanding cellular physiology and potential therapeutic interventions for osmotic imbalances.Expand Specific Solutions04 Medical applications of hypertonic solutions
Hypertonic solutions have various medical applications, including the treatment of edema, intracranial pressure reduction, and as components in certain medical devices. These solutions can help draw excess fluid from tissues or create specific osmotic environments for therapeutic purposes. Their use extends to areas such as wound healing and management of certain medical conditions.Expand Specific Solutions05 Development of novel hypertonic formulations
Ongoing research focuses on developing novel hypertonic formulations with improved efficacy and reduced side effects. These formulations may incorporate additional components to enhance cell protection during dehydration or to target specific cellular processes. Such advancements aim to optimize the use of hypertonic solutions in various biotechnological and medical applications.Expand Specific Solutions
Key Players in Hypertonic Solution Industry
The formulation of hypertonic solutions for cell dehydration is a critical area in biotechnology, currently in a growth phase. The market size is expanding due to increasing applications in cryopreservation, cell therapy, and regenerative medicine. Technologically, the field is moderately mature, with ongoing innovations. Key players like Wisconsin Alumni Research Foundation, The Regents of the University of California, and Magenta Therapeutics are driving advancements. Academic institutions such as Harvard College and Southeast University are contributing significantly to research. Companies like NuChem Pharmaceuticals and Hybio Pharmaceutical are developing commercial applications, indicating a competitive landscape with both established and emerging players.
Wisconsin Alumni Research Foundation
The Regents of the University of California
Innovative Approaches in Osmolyte Selection
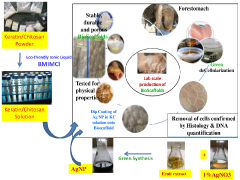
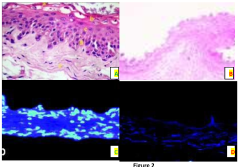
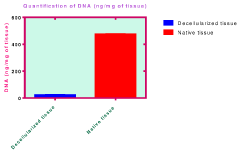
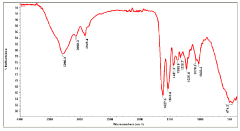
- The use of natural biodetergents like bile juice from animal origin for decellularization, combined with bioactive molecules such as keratin, chitosan, and silver nanoparticles, to enhance the biochemical composition, mechanical strength, and biocompatibility of ECM scaffolds, while providing eco-friendly and non-toxic solutions for sterilization.
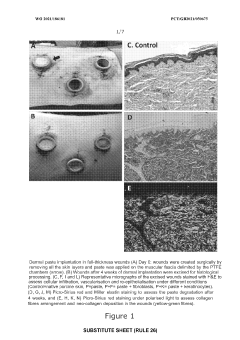
- A combination of decellularised dermal tissue paste and isolated skin cells or a skin cell patch, which can be easily applied to conform to wound topography, reduce fluid accumulation, and promote neocollagen deposition, neovascularisation, and reepithelialisation, creating an optimal wound healing environment.
Regulatory Considerations for Biological Solutions
The formulation of hypertonic solutions for cell dehydration must adhere to strict regulatory guidelines to ensure safety and efficacy in biological applications. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established comprehensive frameworks for the development, manufacturing, and quality control of biological solutions.
These regulatory considerations encompass various aspects of the solution formulation process. Good Manufacturing Practices (GMP) are paramount, requiring manufacturers to implement robust quality management systems, maintain clean and controlled production environments, and employ validated processes. The selection of raw materials and excipients must comply with pharmacopoeial standards, and their sourcing should be traceable and consistent.
Stability testing is a critical regulatory requirement for hypertonic solutions. Manufacturers must demonstrate that the formulation maintains its chemical, physical, and microbiological properties throughout its intended shelf life. This involves conducting long-term and accelerated stability studies under various environmental conditions to determine appropriate storage requirements and expiration dates.
Sterility and endotoxin testing are essential for solutions intended for cell culture or clinical applications. Regulatory agencies mandate specific testing methods and acceptance criteria to ensure the absence of microbial contamination and pyrogenic substances. The use of aseptic processing techniques and validated sterilization methods is often necessary to meet these stringent requirements.
Labeling and packaging regulations must also be carefully considered. Clear and accurate labeling of hypertonic solutions, including composition, concentration, storage conditions, and expiration dates, is crucial for regulatory compliance and user safety. Packaging materials must be compatible with the solution and provide adequate protection against environmental factors that could compromise product integrity.
For hypertonic solutions intended for clinical use, additional regulatory hurdles exist. Preclinical and clinical studies may be required to demonstrate safety and efficacy. The regulatory pathway for approval can vary depending on the intended use, with some solutions classified as medical devices, while others may fall under the category of drugs or biologics.
Manufacturers must also establish pharmacovigilance systems to monitor and report any adverse events associated with the use of their hypertonic solutions. This ongoing surveillance is crucial for maintaining regulatory compliance and ensuring patient safety in the long term.
Environmental Impact of Hypertonic Solutions
The environmental impact of hypertonic solutions used for cell dehydration is a critical consideration in both research and industrial applications. These solutions, typically containing high concentrations of solutes such as salts or sugars, can have significant effects on ecosystems if not properly managed.
One of the primary concerns is the potential for hypertonic solutions to alter local water chemistry when released into aquatic environments. The high solute concentration can disrupt the osmotic balance of natural water bodies, potentially affecting aquatic organisms' ability to regulate their internal water content. This can lead to stress or mortality in sensitive species, particularly in freshwater ecosystems where organisms are not adapted to high salinity conditions.
Furthermore, the disposal of hypertonic solutions may contribute to soil salinization if released onto land. Increased soil salinity can negatively impact plant growth and soil microbial communities, potentially leading to reduced agricultural productivity and altered ecosystem functions in affected areas.
The production and use of hypertonic solutions also have indirect environmental impacts. The manufacturing process of solutes used in these solutions may involve energy-intensive processes and chemical synthesis, contributing to greenhouse gas emissions and potential chemical pollution if not properly controlled.
Water consumption is another significant factor to consider. The preparation of hypertonic solutions often requires purified water, and the increasing demand for such solutions in various industries could strain local water resources, particularly in water-scarce regions.
Efforts to mitigate these environmental impacts are crucial. Implementing proper waste management protocols, including treatment and dilution of hypertonic solutions before disposal, can significantly reduce their ecological footprint. Additionally, exploring alternative formulations that use more environmentally friendly solutes or developing closed-loop systems for solution recycling can help minimize the overall environmental impact.
Research into biodegradable or naturally derived solutes for hypertonic solutions is an emerging area that holds promise for reducing long-term environmental effects. These alternatives could potentially offer similar cell dehydration efficacy while posing less risk to ecosystems upon release.
In conclusion, while hypertonic solutions play a vital role in cell dehydration processes, their environmental impact must be carefully managed. Balancing the technological benefits with ecological considerations is essential for sustainable use of these solutions across various applications.