How to Improve Hypertonic Solution Stability for Long-Term Use?
Hypertonic Solution Stability Background and Objectives
Hypertonic solutions have been a cornerstone in medical treatments for decades, particularly in managing various conditions such as dehydration, electrolyte imbalances, and certain neurological disorders. These solutions, characterized by their higher solute concentration compared to bodily fluids, play a crucial role in osmotic therapy and fluid management. However, the long-term stability of hypertonic solutions has been a persistent challenge in the pharmaceutical industry, affecting their efficacy, safety, and shelf life.
The evolution of hypertonic solution technology has seen significant advancements since its inception. Initially, simple salt solutions were used, but as medical understanding grew, more complex formulations incorporating various electrolytes and osmotic agents were developed. This progression has led to more targeted and effective treatments, but it has also introduced new challenges in maintaining solution stability over extended periods.
Current research in this field aims to address several key objectives. Foremost among these is the enhancement of solution stability without compromising therapeutic efficacy. This involves investigating novel formulation techniques, exploring alternative preservatives, and developing innovative packaging solutions. Another critical goal is to extend the shelf life of hypertonic solutions, which is essential for improving their practicality in clinical settings and emergency situations.
The pharmaceutical industry is also focusing on developing hypertonic solutions that can withstand a broader range of environmental conditions. This is particularly important for global distribution and use in diverse climatic zones. Additionally, there is a growing emphasis on creating more environmentally friendly and cost-effective production methods, aligning with broader sustainability goals in healthcare.
Understanding the molecular interactions within hypertonic solutions is another key research objective. This includes studying how different solutes interact over time, the impact of temperature fluctuations on solution integrity, and the potential for chemical degradation. By gaining deeper insights into these processes, researchers aim to design more stable formulations from the ground up.
The quest for improved hypertonic solution stability is not just a matter of chemical engineering but also involves advancements in packaging technology. Research is underway to develop smart packaging that can monitor and maintain optimal conditions for the solutions, potentially extending their usable life and ensuring quality at the point of use.
As we look towards the future, the objectives for hypertonic solution stability research are becoming increasingly ambitious. There is a push towards developing 'universal' hypertonic solutions that remain stable across a wide range of applications and conditions. This could revolutionize emergency medicine and simplify supply chains in healthcare systems worldwide.
Market Analysis for Stable Hypertonic Solutions
The market for stable hypertonic solutions is experiencing significant growth, driven by increasing demand in various medical and industrial applications. In the healthcare sector, hypertonic solutions are widely used for treating edema, managing intracranial pressure, and as a component in dialysis treatments. The global market for these solutions is expected to expand at a steady rate over the next five years, with a particular focus on long-term stability formulations.
One of the key factors driving market growth is the rising prevalence of chronic diseases that require long-term management with hypertonic solutions. Conditions such as congestive heart failure, liver cirrhosis, and certain kidney disorders often necessitate the use of these solutions for extended periods. As the global population ages and lifestyle-related health issues become more prevalent, the demand for stable hypertonic solutions is projected to increase substantially.
In the industrial sector, hypertonic solutions find applications in food preservation, water treatment, and certain manufacturing processes. The food and beverage industry, in particular, is showing increased interest in stable hypertonic solutions for extending the shelf life of products and improving food safety. This diversification of applications is contributing to the overall market expansion.
Geographically, North America and Europe currently dominate the market for stable hypertonic solutions, owing to advanced healthcare infrastructure and higher healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are expected to witness the fastest growth in the coming years. This growth is attributed to improving healthcare access, rising disposable incomes, and increasing awareness about advanced medical treatments.
The competitive landscape of the stable hypertonic solutions market is characterized by the presence of several key players, including pharmaceutical companies and specialized solution manufacturers. These companies are investing heavily in research and development to improve the stability and efficacy of their products, particularly for long-term use applications. The market is also seeing a trend towards the development of novel formulations that offer enhanced stability without compromising therapeutic efficacy.
Challenges in the market include stringent regulatory requirements, especially for medical-grade solutions, and the need for continuous innovation to address stability issues. However, these challenges also present opportunities for companies that can successfully develop and market improved, long-lasting hypertonic solutions.
In conclusion, the market for stable hypertonic solutions shows promising growth potential, driven by expanding applications in healthcare and industry. The focus on long-term stability is likely to shape product development and market strategies in the coming years, creating opportunities for innovation and market expansion.
Current Challenges in Long-Term Hypertonic Solution Stability
The long-term stability of hypertonic solutions presents several significant challenges that hinder their widespread use and effectiveness in various applications. One of the primary issues is the tendency for these solutions to undergo chemical degradation over time. This process can lead to a reduction in the concentration of active ingredients, potentially compromising the solution's therapeutic efficacy and safety profile.
Osmotic pressure fluctuations pose another critical challenge. Hypertonic solutions rely on maintaining a specific osmotic gradient to function effectively. However, environmental factors such as temperature variations and exposure to light can cause shifts in this delicate balance, leading to unpredictable changes in the solution's osmolarity over extended periods.
Microbial contamination remains a persistent concern, particularly for solutions intended for long-term storage or repeated use. The high solute concentration in hypertonic solutions can create an environment conducive to certain types of microbial growth, necessitating robust preservation methods that do not interfere with the solution's intended function.
The physical stability of hypertonic solutions is also a significant challenge. Precipitation of solutes, especially in highly concentrated solutions, can occur during storage, leading to the formation of crystals or particulate matter. This not only affects the solution's homogeneity but can also pose risks if administered in medical applications.
Container interactions present another layer of complexity. The materials used in packaging hypertonic solutions must be carefully selected to prevent leaching of substances from the container into the solution or adsorption of active ingredients onto container surfaces. These interactions can alter the solution's composition and stability over time.
pH stability is crucial for maintaining the integrity of hypertonic solutions. Many active ingredients are sensitive to pH changes, which can occur gradually during long-term storage. Maintaining a stable pH environment is essential for preserving the solution's efficacy and preventing unwanted chemical reactions.
Lastly, the challenge of maintaining uniform distribution of solutes throughout the solution during long-term storage is significant. Gravitational settling or stratification can occur, particularly in solutions with components of different densities, leading to inconsistencies in concentration and potentially affecting the solution's intended performance.
Addressing these challenges requires a multifaceted approach, combining advanced formulation techniques, innovative packaging solutions, and rigorous quality control measures to ensure the long-term stability and efficacy of hypertonic solutions.
Existing Approaches for Enhancing Solution Stability
01 Stabilization of hypertonic solutions using buffer systems
Buffer systems are employed to maintain the pH and ionic strength of hypertonic solutions, enhancing their stability over time. These systems typically include combinations of weak acids and their conjugate bases, which help resist changes in pH caused by external factors or chemical reactions within the solution.- Stabilization of hypertonic solutions using buffer systems: Buffer systems are employed to maintain the pH and stability of hypertonic solutions. These systems help prevent degradation of active ingredients and ensure the solution remains effective over time. Proper selection of buffer components and concentrations is crucial for maintaining the hypertonic nature of the solution while providing stability.
- Use of preservatives in hypertonic solutions: Preservatives are added to hypertonic solutions to prevent microbial growth and extend shelf life. Careful selection of preservatives is essential to maintain the solution's hypertonicity while providing antimicrobial protection. The type and concentration of preservatives are optimized to ensure compatibility with other ingredients and maintain solution stability.
- Stabilization through packaging and storage conditions: Proper packaging materials and storage conditions play a crucial role in maintaining the stability of hypertonic solutions. Factors such as light protection, temperature control, and moisture barrier properties of packaging materials are considered. Appropriate storage instructions are provided to ensure the solution remains stable throughout its shelf life.
- Formulation techniques for improved stability: Various formulation techniques are employed to enhance the stability of hypertonic solutions. These may include the use of specific excipients, adjustment of ionic strength, and incorporation of stabilizing agents. The formulation process is optimized to ensure that the hypertonic nature of the solution is maintained while improving overall stability.
- Stability testing and quality control measures: Rigorous stability testing and quality control measures are implemented to ensure the long-term stability of hypertonic solutions. These include accelerated stability studies, real-time stability testing, and periodic quality checks. Analytical methods are developed to monitor key parameters such as osmolality, pH, and active ingredient concentration over time.
02 Use of antioxidants to prevent degradation
Antioxidants are added to hypertonic solutions to prevent oxidation and degradation of active ingredients. These compounds scavenge free radicals and inhibit oxidative processes, thereby extending the shelf life and maintaining the efficacy of the solution.Expand Specific Solutions03 Osmolality adjustment for improved stability
The osmolality of hypertonic solutions is carefully adjusted to ensure optimal stability. This involves balancing the concentration of solutes to maintain the desired hypertonicity while preventing precipitation or crystallization of components, which can occur in highly concentrated solutions.Expand Specific Solutions04 Incorporation of stabilizing excipients
Specific excipients are added to hypertonic solutions to enhance their stability. These may include chelating agents, surfactants, or polymers that prevent degradation, maintain homogeneity, or improve the solubility of active ingredients in the hypertonic environment.Expand Specific Solutions05 Temperature-controlled storage and packaging
Proper storage conditions and packaging materials are crucial for maintaining the stability of hypertonic solutions. This includes using temperature-controlled environments and selecting appropriate container materials that are compatible with the solution and protect it from light and moisture.Expand Specific Solutions
Key Players in Hypertonic Solution Industry
The market for improving hypertonic solution stability for long-term use is in a growth phase, driven by increasing demand in healthcare and pharmaceutical sectors. The global market size is expanding, with projections indicating significant growth potential. Technologically, the field is advancing rapidly, with companies like Baxter International, Novo Nordisk, and Novartis leading innovation. These firms are investing heavily in R&D to enhance solution stability and extend shelf life. Emerging players such as Xeris Pharmaceuticals and Yulinghua Technology are also contributing to technological advancements. The competitive landscape is characterized by a mix of established pharmaceutical giants and innovative startups, all striving to develop more stable and effective hypertonic solutions for various medical applications.
Xeris Pharmaceuticals, Inc.
Baxter International, Inc.
Innovative Stabilization Technologies and Patents
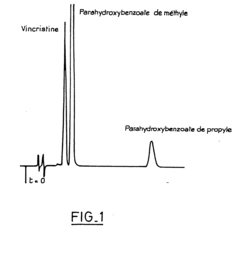
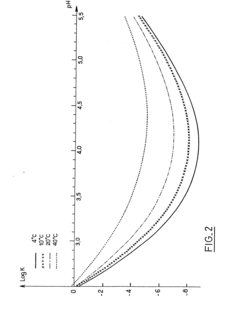
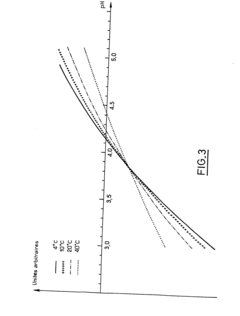
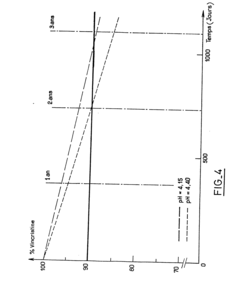
- A stable aqueous solution of vincristine sulphate is formulated with glycocoll to adjust isotonicity, a phosphate buffer to maintain pH at 4.15 ± 0.15, and anti-microbial preservatives, ensuring stability and safety for injection.
- Incorporating histidine or its salts as stabilizers and organic acids or their salts as buffers, adjusting the pH to a range of 5.6 to 6.4, and adding tonicity agents like sodium chloride, sucrose, or glycine to improve the stability of etanercept aqueous formulations, thereby suppressing both aggregate and cleavage product formation.
Regulatory Considerations for Long-Term Use Solutions
Regulatory considerations play a crucial role in the development and commercialization of long-term use hypertonic solutions. These solutions, designed for extended periods of application, must adhere to stringent guidelines set forth by regulatory bodies such as the FDA, EMA, and other international health authorities.
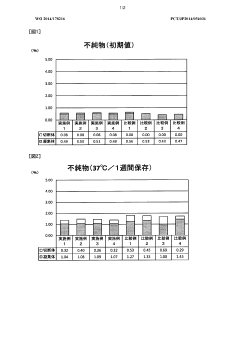
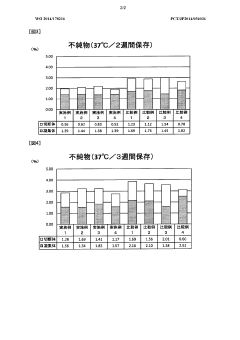
One of the primary regulatory concerns for long-term use solutions is the establishment of comprehensive stability data. Manufacturers must demonstrate that the hypertonic solution maintains its chemical, physical, and microbiological properties throughout its intended shelf life. This typically involves conducting long-term stability studies under various environmental conditions, including temperature, humidity, and light exposure.
Safety and efficacy are paramount in regulatory assessments. For hypertonic solutions intended for long-term use, toxicology studies must be conducted to evaluate potential adverse effects from prolonged exposure. These studies should address both local and systemic toxicity, as well as potential interactions with other medications or medical devices.
The manufacturing process for long-term use solutions is subject to rigorous quality control measures. Good Manufacturing Practices (GMP) must be strictly followed, with particular attention paid to sterility assurance and container closure integrity. Regulatory bodies may require validation of sterilization processes and ongoing monitoring of production facilities.
Labeling and packaging requirements for long-term use solutions are another critical regulatory consideration. Clear instructions for use, storage conditions, and expiration dating must be provided. Additionally, any potential risks or side effects associated with prolonged use should be clearly communicated to healthcare providers and patients.
Post-market surveillance is an essential component of regulatory compliance for long-term use solutions. Manufacturers must implement systems to monitor and report adverse events, as well as conduct periodic safety updates. This ongoing vigilance helps identify any unforeseen issues that may arise from extended use in diverse patient populations.
Regulatory bodies may also require specific clinical trials to support the long-term use of hypertonic solutions. These trials should demonstrate not only the immediate efficacy but also the sustained benefits and safety profile over the intended duration of use. The design of such trials must be carefully considered to address potential ethical concerns related to long-term exposure.
In conclusion, navigating the regulatory landscape for long-term use hypertonic solutions requires a comprehensive approach that addresses stability, safety, manufacturing quality, labeling, post-market surveillance, and clinical evidence. Manufacturers must engage early and often with regulatory authorities to ensure alignment on study designs and data requirements, ultimately facilitating a smoother path to market approval and patient access.
Environmental Impact of Stabilization Methods
The environmental impact of stabilization methods for hypertonic solutions is a critical consideration in the long-term use of these formulations. Traditional stabilization techniques often involve the addition of chemical preservatives or antioxidants, which can have significant ecological consequences when released into the environment. These additives may persist in water systems, potentially affecting aquatic ecosystems and biodiversity.
More environmentally friendly approaches to stabilization are being explored to mitigate these concerns. One promising method involves the use of natural antioxidants derived from plant sources. These compounds, such as polyphenols and flavonoids, can provide effective stabilization while being biodegradable and less harmful to the environment. However, their extraction and production processes must be carefully managed to ensure sustainability and minimize ecological footprints.
Another innovative approach is the development of advanced packaging technologies that can extend the shelf life of hypertonic solutions without the need for additional chemical stabilizers. These may include oxygen-scavenging materials or modified atmosphere packaging, which can reduce oxidation and microbial growth. While these technologies offer environmental benefits by reducing the need for chemical additives, the production and disposal of specialized packaging materials must be considered in the overall environmental assessment.
Nanotechnology-based stabilization methods are also emerging as potential solutions with reduced environmental impact. Nanoencapsulation techniques can protect sensitive components of hypertonic solutions from degradation, potentially eliminating the need for certain stabilizers. However, the long-term environmental effects of nanoparticles are still under investigation, and careful evaluation is necessary before widespread adoption.
The use of physical stabilization methods, such as controlled storage conditions or advanced filtration systems, can also contribute to improved stability with minimal environmental impact. These approaches focus on preventing degradation through environmental control rather than chemical intervention, potentially reducing the release of harmful substances into ecosystems.
As regulatory bodies increasingly emphasize environmental sustainability, the development of eco-friendly stabilization methods for hypertonic solutions is becoming a priority. Research is ongoing to find a balance between effective long-term stability and minimal ecological impact. This includes life cycle assessments of various stabilization methods to comprehensively evaluate their environmental footprints, from raw material sourcing to end-of-life disposal.