How To Make Hypertonic Saline Solution For Nebulizer?
Hypertonic Saline Background and Objectives
Hypertonic saline solutions have been utilized in medical treatments for decades, particularly in respiratory therapy. The use of nebulized hypertonic saline has gained significant attention in recent years due to its effectiveness in treating various respiratory conditions. This technology has evolved from simple saline solutions to more advanced formulations tailored for specific therapeutic purposes.
The primary objective of hypertonic saline solutions for nebulizers is to improve mucociliary clearance and reduce airway inflammation. By increasing the osmolarity of the airway surface liquid, these solutions help to hydrate the airway mucus, making it less viscous and easier to clear. This mechanism is particularly beneficial for patients with conditions such as cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease (COPD).
The development of hypertonic saline solutions has progressed through several stages, from basic sodium chloride mixtures to more sophisticated formulations incorporating additional compounds. Early research focused on determining the optimal salt concentration for therapeutic effects while minimizing potential side effects such as bronchoconstriction.
Current technological trends in this field include the exploration of different salt compositions, the addition of osmotic agents, and the incorporation of mucus-modifying agents. Researchers are also investigating the potential synergistic effects of combining hypertonic saline with other therapeutic agents, such as bronchodilators or anti-inflammatory compounds.
The market demand for hypertonic saline solutions has been steadily increasing, driven by the rising prevalence of chronic respiratory diseases and the growing awareness of the benefits of nebulizer therapy. This has led to a surge in research and development efforts aimed at improving the efficacy and safety of these solutions.
One of the key challenges in this field is balancing the therapeutic benefits of higher salt concentrations with the potential for adverse effects. As such, ongoing research is focused on optimizing the formulation to maximize efficacy while minimizing side effects. Additionally, there is a growing interest in developing personalized hypertonic saline solutions that can be tailored to individual patient needs.
Looking ahead, the future of hypertonic saline solutions for nebulizers is likely to involve more advanced formulations, improved delivery systems, and a better understanding of the underlying mechanisms of action. These advancements will contribute to more effective treatments for respiratory conditions and potentially expand the applications of this technology to other medical fields.
Market Analysis for Nebulizer Solutions
The global market for nebulizer solutions, particularly hypertonic saline solutions, has been experiencing steady growth due to the increasing prevalence of respiratory diseases and the rising adoption of home healthcare devices. The demand for nebulizer solutions is driven by factors such as the growing aging population, rising air pollution levels, and the increasing incidence of chronic respiratory conditions like asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis.
Hypertonic saline solutions for nebulizers have gained significant traction in recent years due to their effectiveness in improving mucociliary clearance and reducing airway inflammation. The market for these solutions is expected to expand further as healthcare providers and patients recognize their benefits in managing various respiratory conditions.
The nebulizer solutions market is segmented based on product type, application, end-user, and region. Hypertonic saline solutions fall under the saline category, which represents a substantial portion of the overall market share. The hospital segment currently dominates the end-user market, but there is a growing trend towards home healthcare, which is expected to drive the demand for portable nebulizers and associated solutions.
North America holds the largest market share in the nebulizer solutions industry, followed by Europe and Asia-Pacific. The United States, in particular, has a well-established market due to its advanced healthcare infrastructure and high prevalence of respiratory diseases. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare access and rising awareness about respiratory health.
Key players in the nebulizer solutions market include pharmaceutical companies specializing in respiratory care, as well as medical device manufacturers. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to strengthen their market position. The competitive landscape is characterized by ongoing research and development efforts to improve the efficacy and convenience of nebulizer solutions, including hypertonic saline formulations.
The COVID-19 pandemic has had a significant impact on the nebulizer solutions market, initially causing a surge in demand due to the respiratory nature of the virus. However, concerns about aerosol-generating procedures have led to some hesitancy in nebulizer use in clinical settings. This has prompted manufacturers to develop new delivery systems and formulations that address these concerns while maintaining therapeutic efficacy.
Looking ahead, the market for nebulizer solutions, including hypertonic saline, is poised for continued growth. Factors such as the increasing focus on personalized medicine, advancements in drug delivery technologies, and the rising demand for home-based respiratory care are expected to drive innovation and market expansion in the coming years.
Current Challenges in Hypertonic Saline Production
The production of hypertonic saline solution for nebulizers faces several significant challenges that impact both the manufacturing process and the end-user experience. One of the primary difficulties lies in maintaining precise concentration levels. Hypertonic saline solutions typically range from 3% to 7% sodium chloride, and even slight deviations can affect therapeutic efficacy and patient safety. Achieving and maintaining this accuracy consistently across large production batches requires sophisticated equipment and rigorous quality control measures.
Another challenge is ensuring the sterility of the solution throughout the production and packaging processes. Nebulizers deliver medication directly to the lungs, making it crucial that the hypertonic saline solution remains free from microbial contamination. This necessitates stringent clean room environments and aseptic manufacturing techniques, which can significantly increase production costs and complexity.
The stability of hypertonic saline solutions over time presents an additional hurdle. These solutions can be prone to crystallization, especially at higher concentrations or when exposed to temperature fluctuations during storage and transport. Manufacturers must develop formulations and packaging solutions that maintain the integrity of the product throughout its shelf life, which often involves the use of stabilizing agents or specialized packaging materials.
Scalability in production is another significant challenge. As demand for hypertonic saline solutions grows, manufacturers must find ways to increase production volume without compromising quality or consistency. This often requires substantial investments in automated production lines and advanced process control systems.
Furthermore, there are regulatory challenges associated with producing hypertonic saline solutions for medical use. Manufacturers must navigate complex approval processes and comply with stringent Good Manufacturing Practice (GMP) guidelines set by regulatory bodies such as the FDA. These regulations cover every aspect of production, from raw material sourcing to final product testing and packaging.
Lastly, the environmental impact of producing and packaging hypertonic saline solutions is becoming an increasingly important consideration. Manufacturers are under pressure to develop more sustainable production methods and eco-friendly packaging solutions, which can be challenging to implement without affecting product quality or increasing costs significantly.
Addressing these challenges requires ongoing research and development efforts, as well as collaboration between manufacturers, healthcare providers, and regulatory bodies to ensure that hypertonic saline solutions for nebulizers meet the highest standards of safety, efficacy, and sustainability.
Existing Methods for Hypertonic Saline Preparation
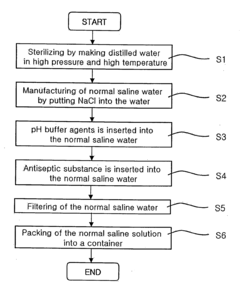
01 Concentration range for hypertonic saline solutions
Hypertonic saline solutions typically have a concentration higher than 0.9% sodium chloride. The concentration can range from 3% to 23.4%, depending on the specific medical application. Higher concentrations are used for more severe conditions or specific treatments, while lower concentrations are used for milder therapies.- Concentration range for hypertonic saline solutions: Hypertonic saline solutions typically have a concentration higher than physiological saline (0.9%). The concentration can range from 3% to 23.4%, depending on the specific medical application. Higher concentrations are used for more severe conditions or specific treatments, while lower concentrations are used for milder cases or maintenance therapy.
- Methods of preparing hypertonic saline solutions: Various methods are used to prepare hypertonic saline solutions, including direct dissolution of sodium chloride in water, dilution of concentrated stock solutions, and use of pre-measured packets or vials. Sterile preparation techniques are often employed to ensure the safety of the solution for medical use.
- Applications of hypertonic saline solutions: Hypertonic saline solutions have diverse medical applications, including treatment of bronchiolitis, cystic fibrosis, cerebral edema, and as a nasal irrigation solution. The concentration used varies depending on the specific condition being treated and the route of administration.
- Delivery systems for hypertonic saline solutions: Various delivery systems are used for administering hypertonic saline solutions, including nebulizers, nasal sprays, intravenous infusion systems, and specialized irrigation devices. The choice of delivery system depends on the intended use and the required concentration of the solution.
- Additives and formulations for hypertonic saline solutions: Hypertonic saline solutions may include additives to enhance their effectiveness or reduce side effects. These can include buffers to adjust pH, preservatives for multi-dose formulations, and other active ingredients such as antimicrobial agents or mucolytics. The specific formulation depends on the intended use and desired properties of the solution.
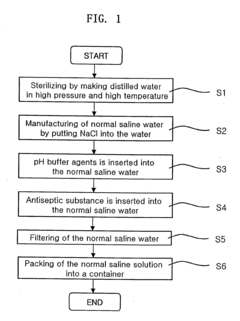
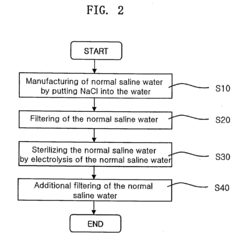
02 Methods of preparing hypertonic saline solutions
Various methods are employed to prepare hypertonic saline solutions, including direct mixing of salt with water, dilution of concentrated stock solutions, and use of specialized equipment for precise concentration control. These methods ensure accurate and consistent preparation of hypertonic saline solutions for medical use.Expand Specific Solutions03 Applications of hypertonic saline solutions
Hypertonic saline solutions have diverse medical applications, including treatment of edema, bronchiolitis, cystic fibrosis, and as a nasal irrigation solution. The concentration of the solution is tailored to the specific medical condition and treatment goals, with higher concentrations used for more severe conditions.Expand Specific Solutions04 Delivery systems for hypertonic saline solutions
Various delivery systems are used for administering hypertonic saline solutions, including nebulizers, nasal sprays, intravenous infusion systems, and specialized irrigation devices. These delivery methods are designed to ensure accurate dosing and effective application of the solution to the target area.Expand Specific Solutions05 Safety considerations and monitoring of hypertonic saline use
The use of hypertonic saline solutions requires careful monitoring of patient response and potential side effects. Safety considerations include proper concentration verification, sterility maintenance, and monitoring of electrolyte balance. Protocols are established to ensure safe administration and to manage any adverse reactions.Expand Specific Solutions
Key Players in Respiratory Therapeutics Industry
The development of hypertonic saline solutions for nebulizers is in a mature stage, with a growing market driven by increasing respiratory disorders. The global nebulizer market, including hypertonic saline solutions, is expected to reach significant size in the coming years. Technologically, the field is well-established, with companies like CHIESI Farmaceutici SpA, Allergan, Inc., and Baxter International, Inc. leading in research and development. These firms, along with others such as Avalyn Pharma, Inc. and Spexis Ltd., are continually refining formulations and delivery systems to enhance efficacy and patient comfort. The competition is focused on improving solution stability, reducing side effects, and developing novel combinations with other therapeutic agents.
CHIESI Farmaceutici SpA
Avalyn Pharma, Inc.
Core Innovations in Saline Solution Technology
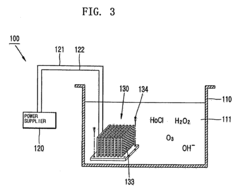
- A method using electrolysis to generate oxidants (ozone, hydrogen peroxide, and hypochlorous acid) for sterilization, combined with a portable contact lens washer that uses a compact electrode unit with projections to concentrate electric charges, allowing for on-site production of NS and effective cleansing of contact lenses.
- A procedure involving the simultaneous addition of phosphoric acid and sodium hydroxide to deionized water, maintaining continuous agitation, to produce a hypertonic saline solution with a 1:2 ratio of bisodium phosphate to monosodium phosphate, which results in a stable and non-irritating pH suitable for therapeutic use.
Regulatory Framework for Nebulizer Solutions
The regulatory framework for nebulizer solutions, particularly hypertonic saline solutions, is a critical aspect of ensuring patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) oversees the regulation of nebulizer solutions as medical devices. These products are typically classified as Class II devices, requiring premarket notification (510(k)) before they can be marketed.
The FDA has established specific guidelines for the manufacturing, testing, and labeling of nebulizer solutions. These guidelines include requirements for sterility, particle size distribution, and chemical composition. Manufacturers must demonstrate that their products meet these standards through rigorous testing and documentation.
In the European Union, nebulizer solutions fall under the Medical Device Regulation (MDR). The MDR requires manufacturers to obtain CE marking for their products, which involves a conformity assessment process to ensure compliance with safety and performance requirements. This process includes risk management, clinical evaluation, and post-market surveillance.
Internationally, the International Organization for Standardization (ISO) has developed standards relevant to nebulizer solutions, such as ISO 27427:2013 for nebulizing systems and components. These standards provide guidance on performance testing and quality control measures.
Specific to hypertonic saline solutions, regulatory bodies often require manufacturers to provide data on osmolality, pH, and sodium chloride concentration. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide monographs for sodium chloride inhalation solution, which serve as reference standards for quality control.
Labeling requirements for nebulizer solutions are stringent and must include clear instructions for use, storage conditions, and any potential side effects. For hypertonic saline solutions, labels must accurately state the concentration and osmolality of the solution.
Regulatory agencies also mandate good manufacturing practices (GMP) for the production of nebulizer solutions. This includes requirements for clean room environments, water quality, and quality control processes throughout the manufacturing and packaging stages.
Post-market surveillance is an essential component of the regulatory framework. Manufacturers are required to monitor the safety and performance of their products after they have been released to the market and report any adverse events or product defects to the relevant regulatory authorities.
Safety and Efficacy Considerations
The safety and efficacy of hypertonic saline solutions for nebulizers are critical considerations in their preparation and use. Hypertonic saline, typically ranging from 3% to 7% concentration, has shown promising results in treating various respiratory conditions, particularly in patients with cystic fibrosis and bronchiectasis. However, its use requires careful attention to potential risks and optimal dosing strategies.
Safety considerations primarily revolve around the risk of bronchospasm, especially in patients with reactive airways. To mitigate this risk, it is recommended to perform a tolerance test before initiating regular treatment. This involves administering a small dose of hypertonic saline and monitoring for adverse reactions. Additionally, pre-treatment with a bronchodilator can help prevent bronchospasm in susceptible individuals.
The osmotic effect of hypertonic saline can cause temporary irritation of the airways, leading to cough and chest tightness. While these effects are generally mild and transient, they should be communicated to patients to ensure compliance and reduce anxiety. Long-term use of hypertonic saline has not shown significant adverse effects on lung function or airway inflammation.
Efficacy considerations focus on the optimal concentration and dosing regimen. Studies have shown that 7% hypertonic saline is generally more effective than 3% in improving mucociliary clearance and lung function. However, the higher concentration may be less tolerable for some patients. A personalized approach, starting with lower concentrations and gradually increasing as tolerated, may optimize both efficacy and adherence.
The frequency of administration also plays a crucial role in efficacy. Twice-daily nebulization is commonly recommended, but some studies suggest that more frequent administration (up to four times daily) may provide additional benefits in severe cases. The duration of nebulization sessions typically ranges from 10 to 15 minutes, depending on the volume of solution used.
To ensure consistent efficacy, proper nebulizer cleaning and maintenance are essential. Contamination of nebulizer equipment can lead to respiratory infections and reduce the effectiveness of the treatment. Patients should be educated on proper cleaning techniques and the importance of regular equipment replacement.
Monitoring of lung function and symptom improvement is crucial for assessing the ongoing efficacy of hypertonic saline therapy. Regular spirometry tests and quality of life assessments can help healthcare providers adjust treatment plans as needed. Additionally, monitoring serum electrolyte levels, particularly in patients with comorbidities, can help ensure the safety of long-term use.